The treatment landscape of acute myeloid leukaemia (AML), which historically has been undertreated and considered a medical emergency with poor prognosis, is becoming full of hope for newly diagnosed patients. With recent clinical trials and drug approvals, a once bleak landscape is being transformed.
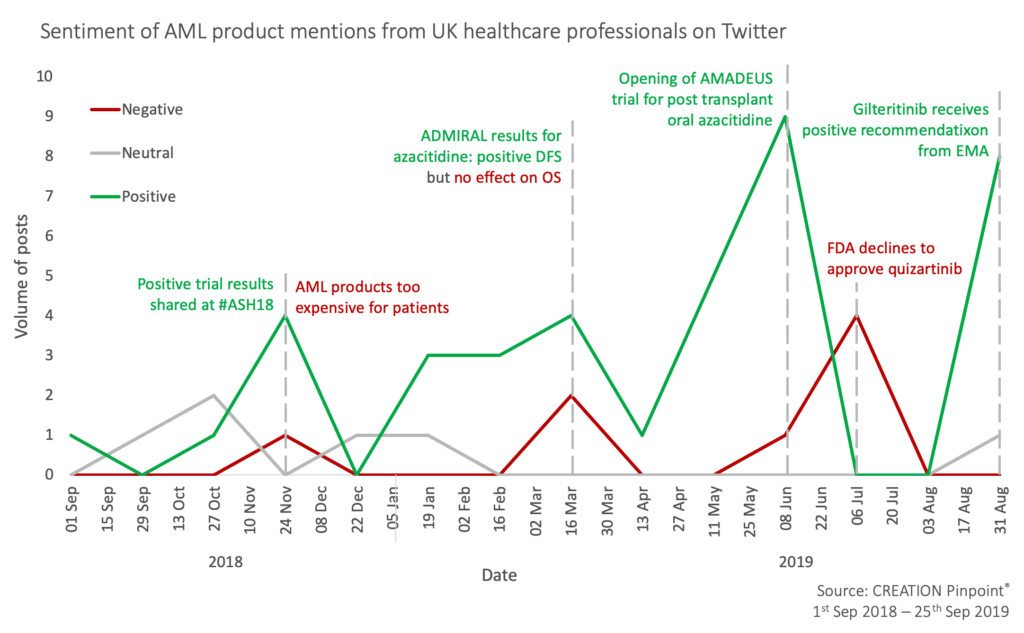
Healthcare professionals (HCPs) have been discussing these changes on social media. In the UK, for example, 10% of posts by HCPs over the past year mentioned specific new treatments for the disease. CREATION.co’s human sentiment analysis of these posts illuminated a positive anticipation for these products.
1. Data for patients over 60
Congress meetings are always a key time for HCPs to respond to trial results of new and existing products. In December 2018, UK HCPs shared data that had been presented during the American Society of Hematology, #ASH18, showing encouraging results. One example was a “promising” trial of venetoclax (AbbVie’s Venclyxto) and idasanutlin (Roche) in AML patients over the age of 60.
#Venetoclax and #idasanutlin in patients 60 years and over with #AML show promising responses; concomitant azole use may require dose reduction. Diarrhoea is a significant adverse effect that can be treated with prophylaxis. #ASH18 pic.twitter.com/BWzy8scaIp
— Prof. Amit Patel 🇬🇧 (@DrAmitPatel) December 4, 2018
While these results were emerging, HCPs in the UK shared a post from David Steensma, a US haematologist-oncologist who highlighted the cost barrier for patients who could only afford novel treatments via clinical trials. The tweet sparked a discussion around cost benefit. Navneet Majhail, another US doctor, suggested bone marrow transplantation will be more cost-effective, while Steensma said plainly that whilst there had been many recent approvals, these will not all necessarily aid patients.
#Gilteritinib cost: $32K per month. Seems like each new approval in #AML is ratcheting up the price; #enasidenib debuted at $24,872/month, #ivosidenib at $26,115. I enrolled patients on all 3 pivotal trials who could not have afforded drugs had they been approved. $ALPMY 😞
— David Steensma, MD (@DavidSteensma) December 13, 2018
Amidst these cautions, more trials were completed and data shared. HCPs often respond on social media to not only new advances in treatment but also disappointments in data. Their understanding of the landscape is shaped broadly by the trial statistics, commentary from their peers and sometimes provocative discussions that unite clinicians from all backgrounds and therapy areas.
2. Maintenance treatment data
One area seeing advancement is maintenance treatment for AML patients post intense chemotherapy or bone marrow transplant. In April, results of a trial investigating azacitidine maintenance were released, headlining an improved disease-free survival (DFS). However, HCPs online quickly noted the DFS survival did not relate to overall survival (OS); they showed their concerns that these results may not affect treatment behaviour.
But not OS- intriguing, 🤔hopefully see the results of QUAZAR – ‘oral AZA’ soon as I don’t think SC AZA is a new standard for maintenance in AML https://t.co/nppJP2zLom
— Mike Dennis (@mikewdennis) April 2, 2019
Updated news in September came of Celgene’s Phase III QUAZAR trial meeting its primary and secondary endpoints for oral-azacitidine post-chemotherapy (OS and relapse-free survival, RFS). HCPs in the UK shared this news but without additional comment.
Following this, UK HCPs were keen to share the opening of the AMADEUS trial for post-transplant oral azacitidine, the first of its kind. The online behaviours of HCPs show their consistent desire for research into new products and evidence that they will be safe and effective for their patients, never fully satiated with the latest release.
👍AMADEUS is the only randomised trial of oral azacitidine-CC486-maintenance in patients allografted for AML and now recruiting through @impactstemcell – the UK’s transplant trial network. Positive read out from AML001 in non-transplant setting therefore of major interest. 👇 https://t.co/VbljPjyMv3
— Charlie Craddock CBE (@charliecraddock) September 17, 2019
3. A new standard of care
Whilst many continually seek further data, one oncologist in London, Sarah Halford, suggested the results of the ADMIRAL trial may call for a new standard of care in Astellas’ Xospata. This bold question exemplifies the strong opinions HCPs are willing to air on social media, asking for and providing a means for group analysis and consideration that may not be feasible offline.
Based on the results of the ADMIRAL trial, presented by Alexander Perl, do we have a new standard of care for FLT3 positive relapsed or refractory #AML ? – #AACR19 pic.twitter.com/V3bp2Z43zJ
— Sarah_Halford (@sarahhalford7) April 2, 2019
Even with new products being added to the AML treatment landscape, there was still bad news for the therapy area. In July, the FDA declined Daiichi Sankyo’s quizartinib for the treatment of FLT3-mutated AML. HCPs were disappointed that another potential new treatment option for patients failed to reach the mark.
Not good news for Quizartinib and AML in general. Concerns expressed about the quality of the data and about adverse events. https://t.co/ff8kV4OIou
— Thomas Lofaro (@LofaroThomas) July 22, 2019
Most recently, an EMA positive recommendation of Astellas’ gilteritinib (Xospata) as a monotherapy gave UK HCPs a renewed hope for their patients in the NHS. The online response showed HCPs were cautious about the possibility of novel treatments, aware of the knowledge that an approval does not immediately equate to a newly accessible drug for patients. Mike Dennis, a hematologist-oncologist from Manchester, commented that the approval may mean UK NHS patients could receive the treatment that previously was not an option.
#gilteritinib This is the first ever approval by EMA for a ‘non intensive targeted’ treatment as monotherapy in #AML. Possibly a step closer for UK NHS patients too? 🤔 https://t.co/68zQHDnDD8
— Mike Dennis (@mikewdennis) September 24, 2019
A renewed hope in AML
2019 has seen a wave of change in the AML treatment landscape, with both successes and failures. HCPs tracked this evolution on social media, celebrating and cautiously anticipating new data and possible options, such as Astellas’ Xospata, and sharing disappointment when products failed to meet hopes, namely Daiichi Sankyo’s quizartinib. As the market changes, HCPs will inevitably continue to vocalise their reactions on social media. The tracking of these reactions allow for the isolated and unprompted voice of HCPs to come to light.
The developments in maintenance therapy were particularly well tracked by UK HCPs. As these advancements continue, they will present a key opportunity for pharmaceutical companies to engage with their HCP customers.
Monthly, CREATION Pinpoint tracks the online HCP response to various pharmaceutical matters including drug approvals and pharma engagement. For bespoke HCP insights, get in touch.
The header image is of ‘human cells with acute myelocytic leukemia (AML) in the pericardial fluid, shown with an esterase stain at 400x’ and was released by the National Cancer Institute.

 By Mary Kangley
By Mary Kangley