
The most discussed EMA approval this month
The first-ever Ebola vaccine was granted EU-wide conditional marketing authorisation on the 11th of November after decades of research, and healthcare professionals (HCPs) could not hide their excitement. The approval of Merck’s vaccine Ervebo was mentioned 147 times by HCPs online, making it the most discussed EMA approval this month.
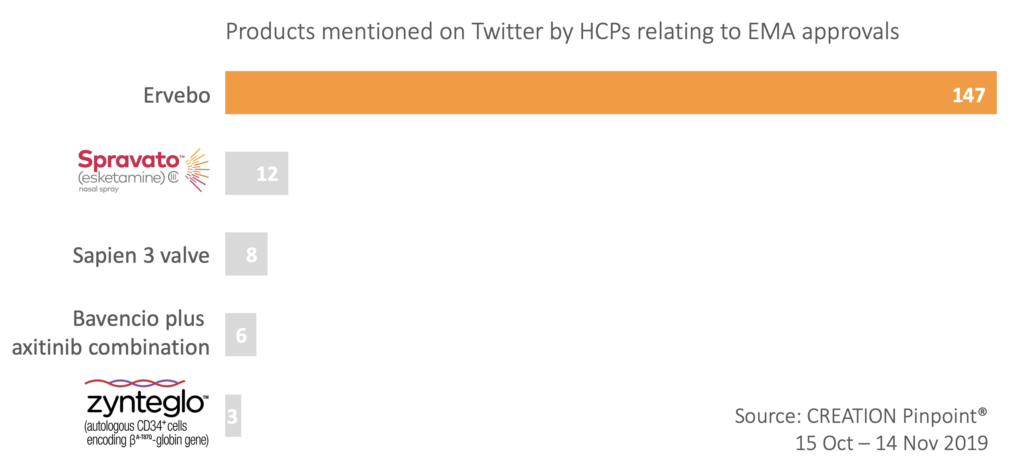
The significance of this approval was described by HCPs as a “landmark moment in the fight against a deadly disease”.
Several HCPs expressed their hope that other regional approvals would soon follow.
The next most mentioned approvals by online HCPs were Janssen’s product esketamine for treatment-resistant major depressive disorder, and Edward Lifesciences’ Sapien 3 transcatheter heart valve system.
We are tracking the HCP reaction to EMA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates, including FDA drug approvals, within the Tracking section of CREATION Knowledge.
Methodology notes:
- Data for this research was analysed from the online Twitter conversations of HCPs talking about EMA approvals in English language (all other languages are available), between October 15th and November 14th 2019.
- Between 15th October and 14th November 195 HCPs posted about EMA approvals more than 200 times all over the world.
 By Laura McIntyre
By Laura McIntyre 
