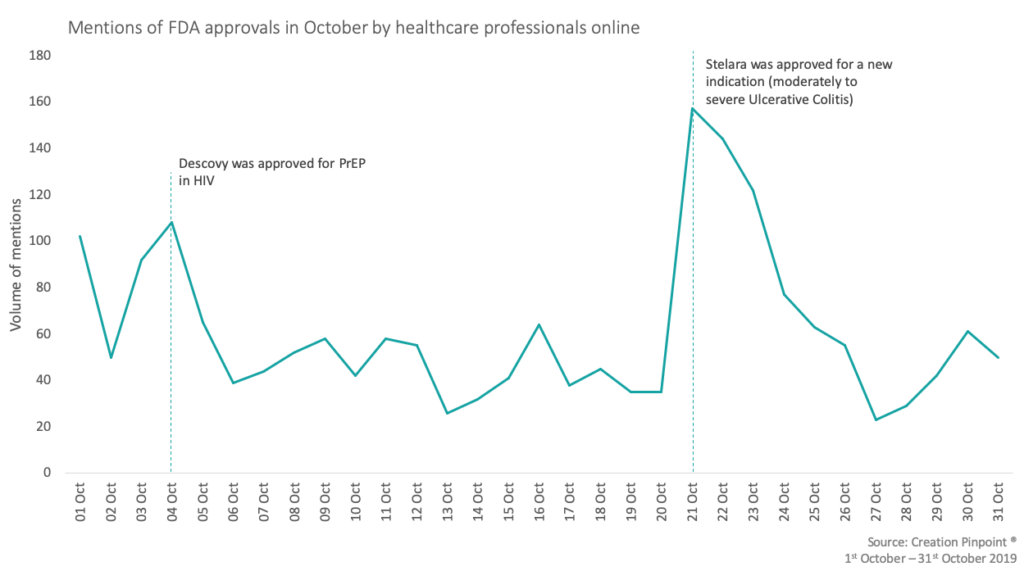
CREATION Pinpoint’s Drug Launch Tracker shows how healthcare professionals (HCPs) respond to drug launch news, as it happens. The FDA approved over 300 submissions within the month of October which included medication for cystic fibrosis, ulcerative colitis and HIV.
The most mentioned FDA approvals by HCPs online
| Brand | Compound(s) | Manufacturer | Brand mentions | Compound mentions | Total mentions |
|---|---|---|---|---|---|
| Descovy | emtricitabine; tenofovir alafenamide fumarate | Gilead Sciences | 59 | 12 | 71 |
| Farxiga | dapagliflozin | Astrazeneca | 7 | 60 | 67 |
| Entresto | sacubitril; valsartan | Novartis | 16 | 46 | 62 |
| Trikafta | elexacaftor, tezacaftor, ivacaftor | Vertex | 56 | 0 | 56 |
| Stelara | ustekinumab | Janssen | 0 | 55 | 55 |
| Xarelto | rivaroxaban | Janssen | 0 | 34 | 34 |
| Reyvow | lasmiditan succinate | Eli Lilly And Co | 0 | 32 | 32 |
| Asenapine | asenapine maleate | Sigmapharm Labs Llc | 11 | 11 | 22 |
| Naftifine | naftifine hydrochloride | Taro | 21 | 0 | 21 |
| Botox | onabotulinumtoxina | Allergan | 19 | 1 | 20 |
| Risperidonea | risperidone | Zydus Pharms Usa Inc | 18 | 1 | 19 |
| Fiasp | insulin aspart | Novo Nordisk | 6 | 8 | 14 |
| Liletta | levonorgestrel | Medicines360 | 13 | 0 | 13 |
| Secuado | asenapine | Hisamitsu Pharm Co | 0 | 11 | 11 |
| Amzeeq | minocycline | Foamix Pharma Inc | 3 | 8 | 11 |
| Zejula | niraparib tosylate | Tesaro Inc | 0 | 11 | 11 |
| Xgeva | denosumab | Amgen | 10 | 0 | 10 |
| Subsys | fentanyl | Btcp Pharma | 7 | 3 | 10 |
| Xtampza | oxycodone | Janssen | 10 | 0 | 10 |
| Suboxone | buprenorphine hydrochloride; naloxone hydrochloride | Indivior Inc | 1 | 8 | 9 |
| Vytorin | ezetimibe; simvastatin | Msd Intl | 8 | 0 | 8 |
| Biorphen | phenylephrine hydrochloride | Sintetica Sa | 3 | 5 | 8 |
| Quzyttir | cetirizine hydrochloride | Jdp Therapeutics Inc | 0 | 7 | 7 |
| Xofluza | baloxavir marboxil | Genentech Inc | 0 | 7 | 7 |
| Baxdela | delafloxacin meglumine | Melinta | 2 | 5 | 7 |
| Ranexa | ranolazine | Gilead Sciences | 7 | 0 | 7 |
| Aklief | trifarotene | Galderma Research And Development Llc | 3 | 3 | 6 |
| Lynparza | olaparib | Astrazeneca | 0 | 6 | 6 |
| Carbidopaa | carbidopa | Aurobindo Pharma Ltd | 0 | 6 | 6 |
| Sildenafil | sildenafil citrate | Perrigo R And D | 5 | 0 | 5 |
The most mentioned approval this month, with 71 mentions, was Gilead’s Descovy (emtricitabine + tenofavir). This was approved for the use in HIV prophylaxis for men and transgender women however, HCPs raised concerns and suggested a need for more inclusive trials. Even with this concern HCPs are positive towards the approval saying that it is a ’big win for the HIV community’ and ‘BIG news for patients’.
The second most mentioned product in October, was AstraZeneca’s SGLT-2 inhibitor Farxiga (dapagliflozin) for their new indication to reduce heart failure (HF) in type 2 diabetes patients. This news was shared 67 times by HCPs during October with one pharmacist, Diana Isaacs in Cleveland, Ohio, sharing her excitement for the approval in a post well engaged by her peers.
HCPs were positive about the approval of Novartis’ Entresto (sacubitril/valsartan) for use in treating dilated cardiomyopathy (DCM) and HF in children describing it as ‘a new hope’. Collectively, they shared this news 62 times, making it the third most mentioned product this month.
We are tracking the HCP reaction to FDA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates within the Tracking section of CREATION Knowledge, or by signing up to our monthly eJournal.
READ LAST MONTH’S FDA APPROVAL TRACKER:
Methodology notes:
- Data for this research was analysed from the online Twitter conversations of HCPs talking about FDA approvals in English language (all other languages are available), between October 1st and October 31th 2019.
- Between October 1st and October 31th 2019, 1,459 HCPs posted about FDA approvals 1,904 times all over the world.
- HCPs mention brand and compound names when discussing FDA approvals. Approvals may include multiple compounds.