The beginning of 2018 saw healthcare professionals (HCPs) in the US frustrated at the increasing price tag for AbbVie’s blockbuster drug Humira (adalimumab). The drug is a biologic used to treat a variety of inflammatory diseases and is the biggest selling prescription drug in the world. Humira has become a household name: it made $18 billion in 2017, sold to treat ten different inflammatory indications. Humira sales make up two-thirds of AbbVie’s revenue, so it is perhaps unsurprising that, with patent expiries looming, AbbVie has been making licensing deals that will maximise its revenues. But how have the company’s tactics – which have been described as ‘patent abuse’ – resonated with those prescribing Humira? And what could this mean for AbbVie in the long run?
By studying the online conversation of US HCPs, it was seen that whilst doctors continue to prescribe Humira, they are not satisfied with AbbVie’s tactics to maintain their market share in the US.
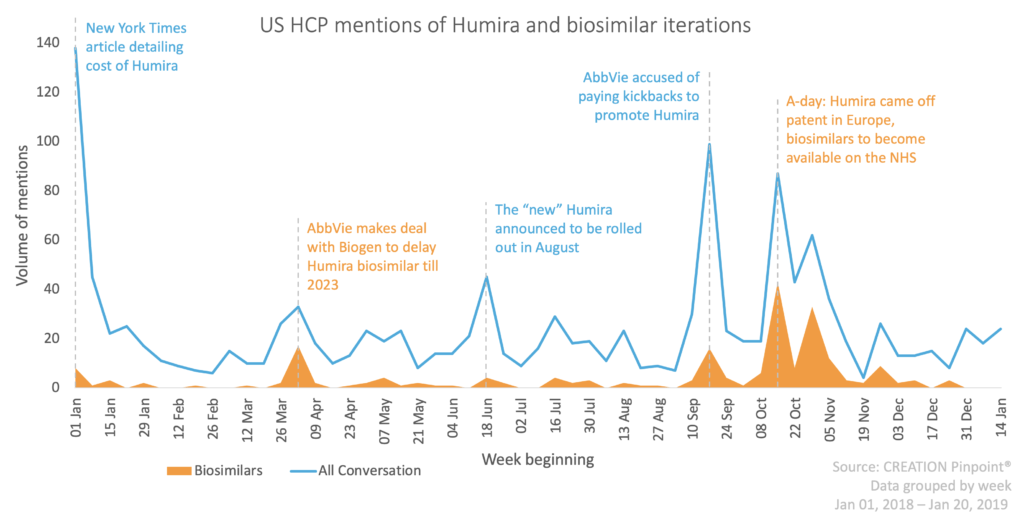
One article in The New York Times (published 6 January 2018) highlighted the increasing cost of Humira in the US, and this article was shared online by 96 HCPs. Some of these individuals added their own take on this information, highlighting their frustration and dismay, often directed at AbbVie. HCPs believe AbbVie is abusing their position of power: pharmacist John Armitstead shared the article adding a comment “because they can…”.
With Humira costing $38,000 per patient per year in the US according to the New York Times article, many HCPs focused on international price discrepancies, calculating that it may be cheaper to fly to another country to buy the drug there, than to purchase it in the US.
This conversation was fueled by The New York Times sharing the information from their article; this specific tweet was retweeted by eight HCPs in the US.
One HCP who was particularly active in the conversation was rheumatologist Dr John Cush. He tweeted 37 times about adalimumab in 2018, sharing trial and study results, approval information and other drug news. He posted The New York Times article, highlighting the price differences internationally. He received considerable engagement from other HCPs on Twitter and has over 6,000 followers. Key influencers such as Dr Cush have the ability to shape online opinion and conversation, which in the face of controversy can have a lasting effect on overall outcome.
In April 2018, AbbVie won a patent settlement (one of many alike) against Biogen prohibiting them from launching their adalimumab biosimilar until 2023. Our study of the HCP online conversation has shown that AbbVie’s aggressive steps to prolong their monopoly in this space have been poorly received by HCPs in the US, with HCPs indicating a sense of injustice that cheaper and similarly effective products are being withheld from the patients who most need them. Could what looks like a short term financial win for AbbVie develop into a crisis that will have far-reaching, long term consequences for AbbVie’s reputation amongst prescribers?
One rheumatologist in New Orleans vocalised her concerns online, citing “patent fighting” as AbbVie’s attempt to protect their own monopoly.
Humira came off patent in Europe on 17 October 2018 (dubbed the highly anticipated “A-day”), heralding lower prices and a wider range of treatment options for European patients. This crash in EU prices served to exacerbate the aggravation of US HCPs and they continued to discuss AbbVie’s so called “patent abuse” in the US throughout the rest of the year.
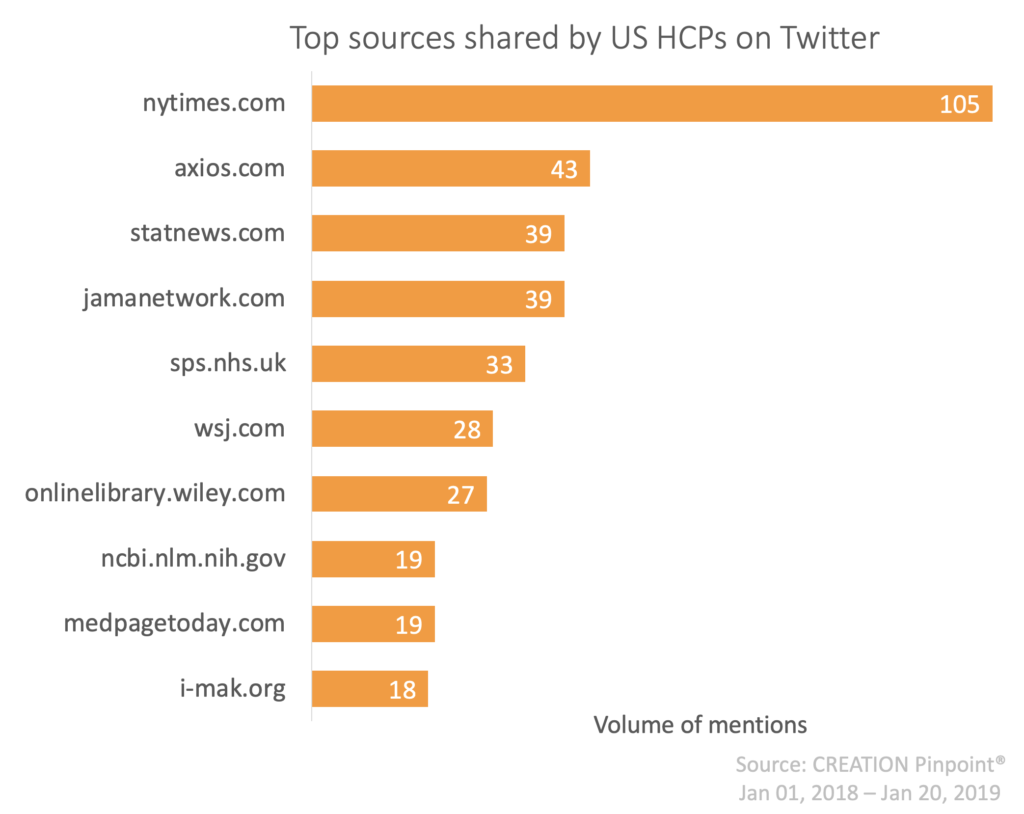
In their tweets about Humira and its biosimilars, 62% of HCP comments contained a link to an article, suggesting that shareable media played a key role in influencing HCP opinion. Their top shared sources (below) spanned from mainstream media outlets to medical journals and served to reinforce HCP negativity.
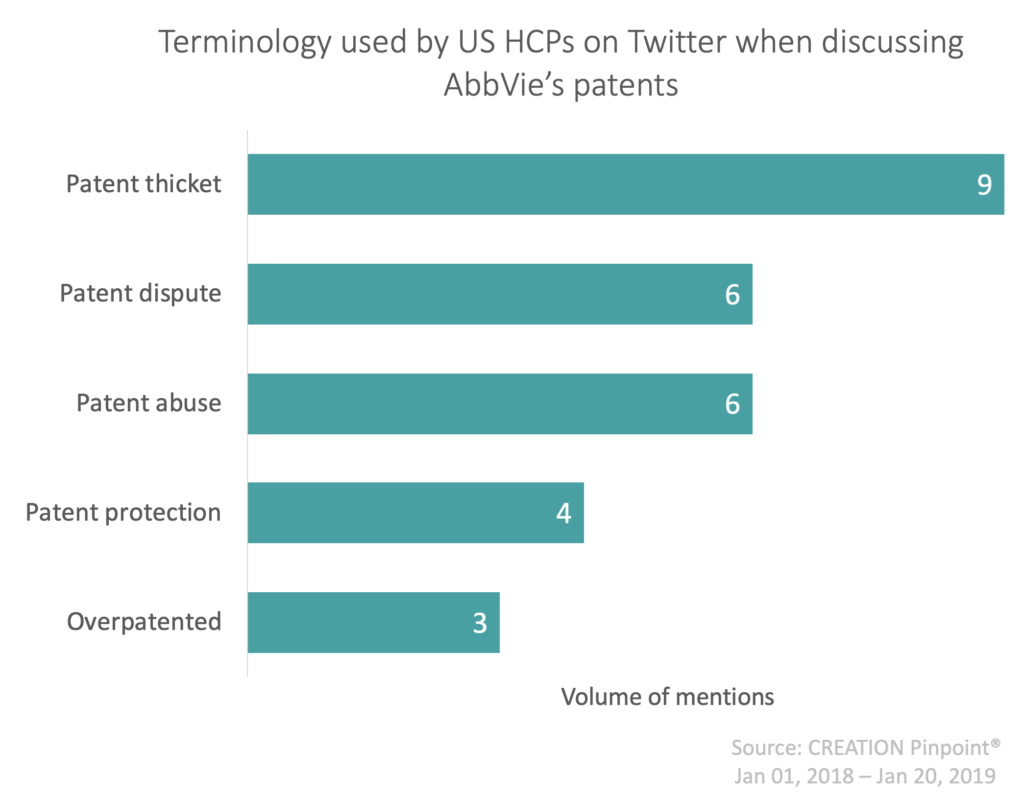
Here, the phrase “patent thicket” was taken from a STAT article which discussed AbbVie’s alleged thwarting tactics. The same website, amongst others, was cited when AbbVie’s reputation took another hit in September when facing a lawsuit from the State of California for paying kickbacks.
This story questioning ethics was shared over 30 times by HCPs in the US.
The HCP online conversation indicates that there are some significant challenges for AbbVie. Yet all hope is not lost for Humira: despite the negative press and the frustration from HCPs about the high price tag of the drug, HCPs continue to acknowledge Humira’s efficacy, and prescribing doctors trust it for the treatment of their patients.
So what next for AbbVie? There is a ray of light in the online conversation: treating chronic conditions such as inflammatory bowel disease and rheumatoid and psoriatic arthritis is no mean feat, so finding a product that works is enough for many doctors and patients alike.
There is still space to celebrate Humira’s efficacy, and the 2018 citrate-free modifications of the drug, which could make a significant improvement to patient quality of life, have as yet been little discussed online. Whilst AbbVie might be overly bold to build a corporate campaign around these strengths given the current HCP backlash around patents, they might consider whether influential HCPs or indeed patients could play a role in re-opening the online dialogue around the benefits of treating with Humira.
A treatment like Humira does more than just resolve physical symptoms for patients suffering with chronic disease; it can positively change their whole life. For AbbVie to stand any chance of retaining market share post 2023, they must do more than just assert the clinical benefits of their product; rather, they need to reconnect with the life-changing impact of Humira, and rebuild HCP trust in AbbVie’s fundamental values – including compassion for people, and commitment to integrity.
 By Mary Kangley
By Mary Kangley 



