Even though quieter during the holidays, the online HCP conversation relating to EMA approvals still spiked following key EMA decisions.
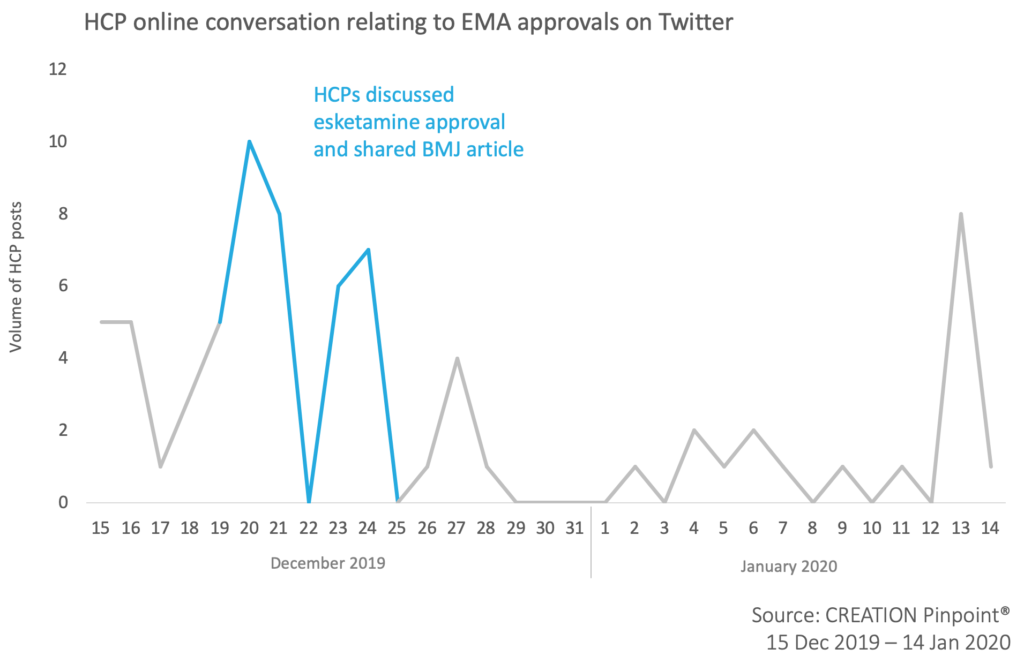
HCPs quickly responded to Janssen’s Spravato (esketamine) receiving EMA approval for treatment-resistant major depressive disorder in adults. They shared a BMJ article reporting the news and a press release from Business Wire.
BMJ reflects the criticism of esketamine's hasty approval by EMA https://t.co/XCFyN1avWU
— Dr Joanna Moncrieff (@joannamoncrieff) December 23, 2019
Out of 37 product-related mentions over the time period, the Spravato approval was mentioned 21 times.
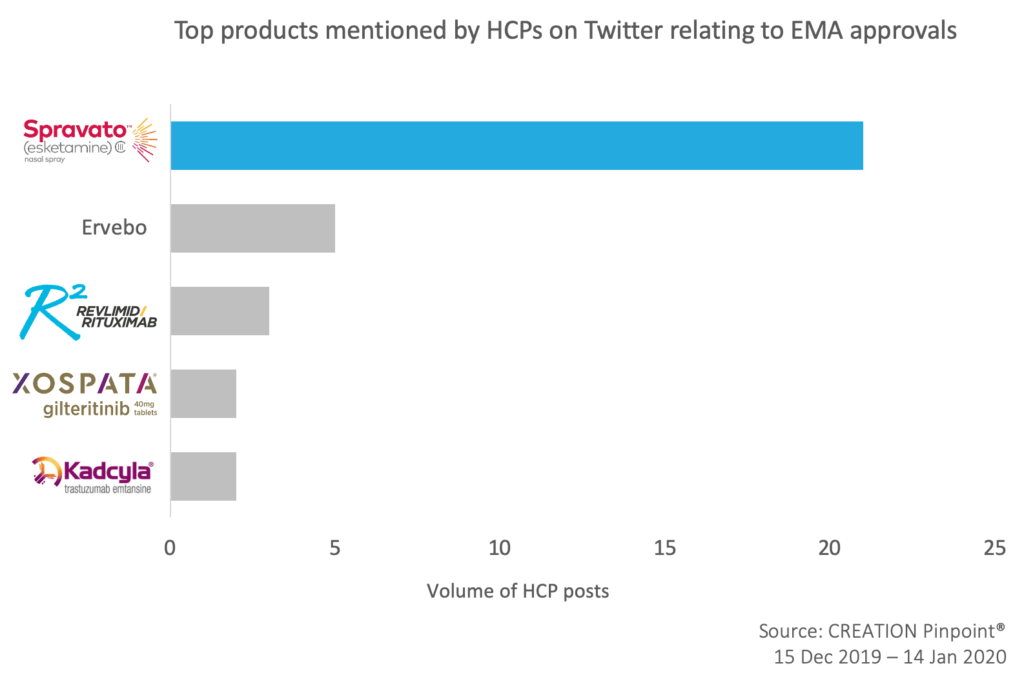
Other approvals discussed by HCPs included looking back at Merck’s Ebola vaccine Ervebo approval that happened back in October and BMS Revlimid (lenalidomide) combination with rituximab for the treatment of relapsed or refractory follicular lymphoma.
HCPs in UK foresee challenges while awaiting EMA approvals
HCPs also showed anticipation for some of the novel therapies being approved by EMA in the near future. UK HCPs stated that the UK needs Trikafta (elexacaftor, ivacaftor and tezacaftor), a new breakthrough therapy for cystic fibrosis, as soon as it is approved in Europe, and wondered how Brexit is going to affect the access to the medicine.
The UK needs #Trikafta as soon as we get EMA approval! https://t.co/9UVeWaENcH
— Rachel cole (@RacheyCole) January 11, 2020
Another anticipated EMA approval is Enhertu (trastuzumab deruxtecan), a metastatic breast cancer treatment by AstraZeneca. HCPs encouraged one another that the EMA approval will soon follow, but admitted there may be a challenge getting it on the NHS.
We are tracking the HCP reaction to EMA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates, including FDA drug approvals, within the Tracking section of CREATION Knowledge or by signing up our monthly newsletter.
READ LAST MONTH’S EMA APPROVAL TRACKER:
New EMA approval in rheumatoid arthritis catches HCP attention
Methodology notes:
- Data for this research was analysed from the online Twitter conversations of HCPs talking about EMA approvals in English language (all other languages are available), between December 15th 2019 and January 14th 2020.
- Between December 15th 2019 and January 14th 2020, 59 HCPs posted relating to EMA approvals 76 times all over the world.
 By Laura McIntyre
By Laura McIntyre