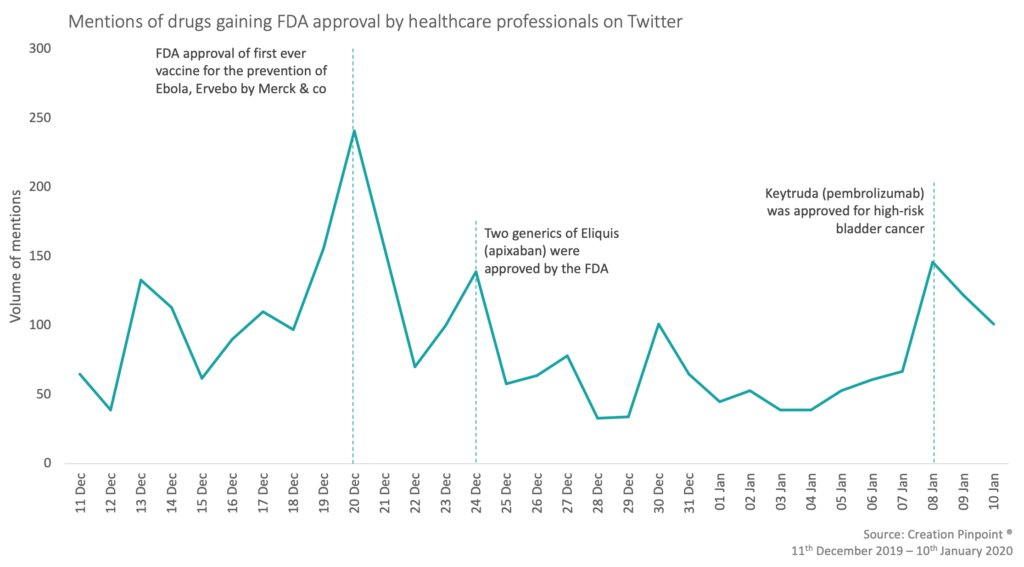
CREATION Pinpoint’s Drug Launch Tracker shows how healthcare professionals (HCPs) respond to drug launch news, as it happens. The FDA approved over 339 submissions between 11 December 2019 – 10 January 2020, which included ebola, high risk bladder cancer and to reduce cardiovascular risk.
The most mentioned FDA approvals by HCPs online
| Brand | Compound(s) | Manufacturer | Brand mentions | Compound mentions | Total mentions |
|---|---|---|---|---|---|
| Vascepa | icosapent ethyl | Amarin Pharms | 100 | 78 | 178 |
| Keytruda | pembrolizumab | Merck & co | 3 | 101 | 104 |
| Lynparza | olaparib | Astrazeneca Pharms | 23 | 61 | 84 |
| Dayvigo | lemborexant | Eisai Inc | 36 | 22 | 58 |
| Xtandi | enzalutamide | Astellas | 24 | 31 | 55 |
| Ubrelvy | ubrogepant | Allergan | 19 | 35 | 54 |
| Enhertu | fam-trastuzumab deruxtecan-nxki | Daiichi Sankyo | 30 | 11 | 41 |
| Vyondys 53 | golodirsen | Sarepta Therapeutics Inc | 15 | 12 | 27 |
| Xeljanz | tofacitinib citrate | Pfizer | 23 | 0 | 23 |
| Ayvakit | avapritinib | Blueprint Medicines Corp | 5 | 18 | 23 |
| Padcev | enfortumab vedotin-ejfv | Astellas | 12 | 8 | 20 |
| Fiasp | insulin aspart | Novo | 5 | 9 | 14 |
| Caplyta | lumateperone tosylate | Intra-Cellular Therapies Inc | 10 | 0 | 10 |
| Genosyl | nitric oxide | Vero Biotech | 5 | 5 | 10 |
| Arazlo | tazarotene | Dow Pharm | 1 | 6 | 7 |
| Sertraline Hydrochloride | sertraline hydrochloride | Mylan | 4 | 0 | 4 |
| Duloxetine Hydrochloride | duloxetine hydrochloride | Aurobindo Pharma Ltd | 3 | 0 | 3 |
| Gilenya | fingolimod hydrochloride | Novartis | 3 | 0 | 3 |
| Pregabalin | pregabalin | Cspc Ouyi | 3 | 0 | 3 |
| Capecitabine | capecitabine | Hikma | 1 | 1 | 2 |
| Lamotrigine | lamotrigine | Alembic Pharms Ltd | 1 | 1 | 2 |
| Tiglutik Kit | riluzole | Italfarmaco Spa | 1 | 0 | 1 |
| Nouress | cysteine hydrochloride | Avadel Legacy | 1 | 0 | 1 |
The most mentioned FDA approval was Vascepa® (icosapent ethyl) with 178 mentions. This product was approved to help reduce the risk of cardiovascular events with a third of the mentions by HCPs likening it to omega-3 or fish oil. One osteopathic physician took this further stating that a non-FDA approved alternative has been around for thousands of years, “It’s called fish”. A tweet by cardiologist Dr. Martha Gulati, referencing her blog explaining the difference between fish oils, was shared by 17 HCPs.
Please share my blog with your patients: Martha Moments-
FDA Approval of Icosapent Ethyl (#Vascepa)- All fish oils are not created equal.@CardioSmart @DLBHATTMD @AnumSaeedMD @cshekarMD @mmamas1973 @hvanspall @cardioPCImom @mpob @HeartOTXHeartMD https://t.co/Yq69yxIE3W— Dr. Martha Gulati ♥️🫀❤️🩹🇨🇦 (@DrMarthaGulati) December 16, 2019
Keytruda® (pembrolizumab), was approved for the treatment of Bacillus Calmette-Guerin (BCG) – unresponsive high risk patients with bladder cancer. This was the second most mentioned approval by HCPs with 104 mentions. HCPs were predominantly sharing a positive view towards this approval saying that it is ‘fantastic news’ and ‘another landmark for bladder’. The most shared tweet was authored by oncologist Tom Powles, sharing that patients generally want to avoid a cystectomy and with this approval that is now possible.
https://t.co/RGGyKfHtWh pembrolizumab is FDA approved in BCG rrefractory NMIBC with CIS. 40% respond (CR), 50% of which last a year. The potential to avoid cystectomy is a priory for patients. @Uromigos pic.twitter.com/HDy4Y454Kh
— Tom Powles (@tompowles1) December 17, 2019
We are tracking the HCP reaction to FDA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates within the Tracking section of CREATION Knowledge, or by signing up to our monthly eJournal.
READ LAST MONTH’S FDA APPROVAL TRACKER:
FDA Tracker: Oxbryt and Tecentriq discussed the most by healthcare professionals in November
Methodology:
- Data for this research was analysed from the online Twitter conversations of HCPs talking about FDA approvals in English language (all other languages are available), between December 11th 2019 and January 10th 2020.
- Between December 11th 2019 and January 10th 2020, 878 HCPs shared posts about FDA approvals 2,731 times all over the world.
- The table refers only to approvals that occurred between December 11th 2019 and January 10th 2020. Mentions of approvals before December 11th were not included.
- HCPs mention brand and compound names when discussing FDA approvals. Approvals may include multiple compounds.
