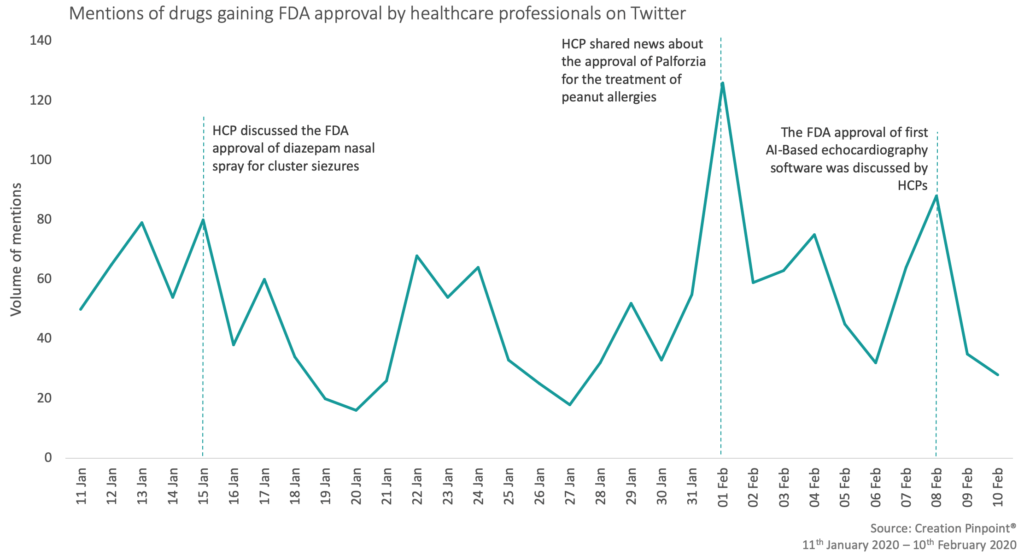
CREATION Pinpoint’s Drug Launch Tracker shows how healthcare professionals (HCPs) respond to drug launch news, as it happens. The FDA approved over 390 submissions between 11 January 2020 – 10 February 2020, which included medications that reduce the risk of cardiovascular disease, block the effects of opioids and improve glycemic control in type 2 diabetes patients.
The most mentioned FDA approvals by HCPs online
| Brand | Compound(s) | Manufacturer | Brand mentions | Compound mentions | Total mentions |
|---|---|---|---|---|---|
| Ozempic | semaglutide | Novo Nordisk | 26 | 45 | 71 |
| Rybelsus | semaglutide | Novo Nordisk | 24 | 24 | 48 |
| Tepezza | teprotumumab-trbw | Horizon Therapeutics Ireland | 14 | 9 | 23 |
| Trijardy Xr | empagliflozin | Boehringer Ingelheim | 15 | 3 | 18 |
| Dificid | fidaxomicin | Cubist Pharms Llc | 3 | 5 | 8 |
| Colchicine | colchicine | Granules Pharms | 3 | 3 | 6 |
| Jardiance | empagliflozin | Boehringer Ingelheim | 4 | 0 | 4 |
| Sublocade | buprenorphine | Indivior Inc | 1 | 2 | 3 |
| Tazverik | tazemetostat hydrobromide | Epizyme Inc | 3 | 0 | 3 |
| Ajovy | fremanezumab-vfrm | Teva Pharms Usa | 2 | 0 | 2 |
| Monoferric | ferric derisomaltose | Pharmacosmos As | 0 | 2 | 2 |
| Pemfexy | pemetrexed | Eagle Pharms | 1 | 1 | 2 |
| Sabril | vigabatrin | Lundbeck Pharms Llc | 1 | 1 | 2 |
| Sildenafil Citrate | sildenafil citrate | Amneal Pharms | 1 | 1 | 2 |
| Testosterone | testosterone | Lupin Ltd | 1 | 1 | 2 |
| Epipen | epinephrine | Mylan Speciality Lp | 1 | 0 | 1 |
| Rituxan | rituximab | Genentech | 0 | 1 | 1 |
This month Novo Nordisk’s Ozempic and Rybelsus (semaglutide) were the top two products mentioned regarding their FDA approval. HCPs shared the news that Ozempic has been approved in a new indication and Rybelsus has been given additional prescribing details. These have both been approved for the risk reduction of major adverse cardiovascular events, such as heart attack and stroke, in patients with type 2 diabetes (T2D). Christopher Cannon, a cardiologist in Boston, shared that the approval of Ozempic is a huge advance for T2D management which was then shared by four HCPs.
FDA Approves CVD Benefit for Once-Weekly Semaglutide – a huge advance for DM manatement!
https://t.co/jKQnKUWttE via @medscape— Christopher Cannon, M.D. 🇺🇦 (@cpcannon) January 17, 2020
Horizon Therapeutics’ Tepezza (teprotumumab), the first and only FDA approved medicine for thyroid eye disease, was the third most mentioned product this month with 23 HCP mentions.
FDA Approves TEPEZZA (teprotumumab) for the Treatment of Thyroid Eye Disease (TED).
It is the first and only FDA-approved medicine for TED.#معلومة_طبية— Dr.Hazem Aljumah د.حازم الجمعة (@Dr_Hazem_) January 23, 2020
We are tracking the HCP reaction to FDA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates within the Tracking section of CREATION Knowledge, or by signing up to our monthly eJournal.
READ LAST MONTH’S FDA APPROVAL TRACKER:
FDA Tracker: HCPs excited for Keytruda® approval but share reservations about Vascepa®
Methodology notes:
- Data for this research was analysed from the online Twitter conversations of HCPs talking about FDA approvals in English language (all other languages are available), between January 11th 2020 and February 10th 2020.
- Between January 11th 2020 and February 10th 2020, 878 HCPs shared posts about FDA approvals 1,572 times all over the world.
- The table refers only to approvals that occurred between January 11th 2020 and February 10th 2020. Mentions of approvals before January 11th were not included.
- HCPs mention brand and compound names when discussing FDA approvals. Approvals may include multiple brands and compounds.
