CREATION Pinpoint’s Drug Launch Tracker shows how healthcare professionals (HCPs) respond to drug launch news, as it happens.
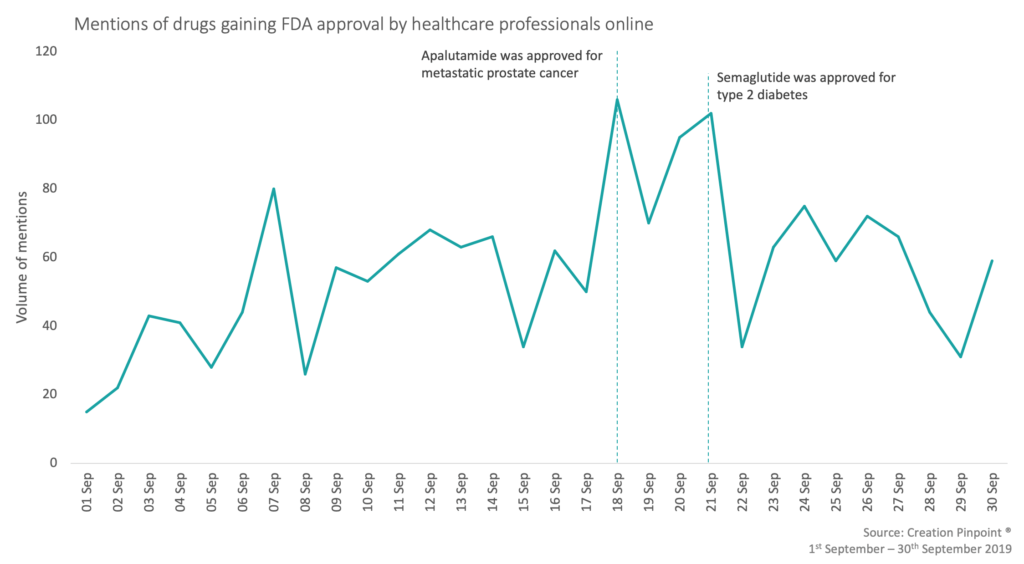
During the month of September, there were over 140 submissions that were approved by the FDA. These approvals ranged from treatments for diabetes, prostate cancer, blood screening and AI technology advances amongst others. With these new treatments, screening options or technology advances gaining approval, 1,280 HCPs shared the news and their views online in 1,744 social media posts.
The most mentioned FDA approvals by healthcare professionals online
| Brand name | Compound name | Manufacturer | Brand mentions | Compound mentions | Total mentions |
|---|---|---|---|---|---|
| Rybelsus | semaglutide | Novo Nordisk | 36 | 82 | 118 |
| Erleada | apalutamide | Janssen | 11 | 83 | 94 |
| Invokana | canagliflozin | Janssen | 19 | 50 | 69 |
| Ofev | nintedanib | Boehringer Ingelheim | 12 | 31 | 43 |
| Darzalex | daratumumab | Janssen | 13 | 25 | 38 |
| Nicotine Polacrilexa | nicotine | Pand L Dev LLC | 17 | 17 | 34 |
| Keytruda | pembrolizumab | Merck & Co | 6 | 18 | 24 |
| Ibsrela | tenapanor | Ardelyx | 4 | 15 | 19 |
| Lenvima | lenvatinib | Merck & Co with Eisai | 4 | 14 | 18 |
| Buprenorphine Hydrochloridea | buprenorphine | Actavis Elizabeth | 8 | 8 | 16 |
| Nucalabla | mepolizumab | GSK | 0 | 11 | 11 |
| Pregabalina | pregabalin | Pfizer | 0 | 9 | 9 |
| Dapagliflozina | dapagliflozin | BMS | 0 | 8 | 8 |
| Tramadol Hydrochloridea | tramadol | Sun Pharmaceuticals | 4 | 4 | 8 |
| Pifeltro | doravirine | Merck & Co | 5 | 3 | 8 |
| Narcan | naloxone | Adapt Pharma | 0 | 7 | 7 |
| Paxil Cr | paroxetine | Apotex Technologies | 0 | 4 | 4 |
| Heparin Sodium | heparin | Pfizer | 2 | 2 | 4 |
| Mydayis | amphetamine | Shire | 0 | 3 | 3 |
| Lamivudinea | lamivudine | Mylan Labs | 0 | 3 | 3 |
| Rituxan | rituximab | Genetech | 0 | 3 | 3 |
| Lamivudinea | lamivudine | Mylan Labs | 0 | 3 | 3 |
| DTG/FTC/TAF | tenofovir | Mylan Labs | 0 | 3 | 3 |
| Paragard T 380 | copper | CooperSurgical | 0 | 2 | 2 |
| Vivitrol | naltrexone | Alkermes | 0 | 2 | 2 |
| Metformin Hydrochloridea | metformin | Sun Pharma | 1 | 1 | 2 |
| Aczone | dapsone | Almirall | 1 | 1 | 2 |
| Fenofibrate (Micronized) | fenofibrate | Cipla | 1 | 1 | 2 |
| Zulresso | brexanolone | Sage Therap | 0 | 1 | 1 |
| Zantac 75 | ranitidine | Sanofi US | 0 | 1 | 1 |
| Hydrocodone Bitartrate And Acetaminophena | acetaminophen | Wes Pharma Inc | 0 | 1 | 1 |
As you can see from the table above, HCPs often mentioned the compound more than the brand name when discussing FDA approvals. This is consistent with what we see across product mentions in general by HCPs online.
Within the top five products discussed by HCPs, we can see that doctors were the most active. However, more role types spoke about Rybelsus® and Invokana®, including pharmacists, due to a wide range of HCPs engaging with patients around these products.
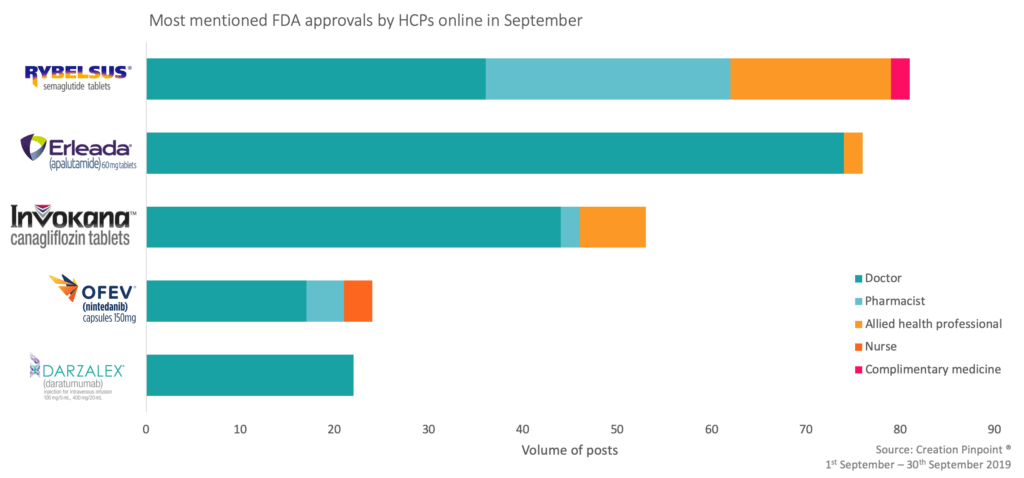
‘Game changing week’ and ‘majorly shake things up’ were some of the phrases used by HCPs to describe the approval of the first oral GLP-1 receptor agonist in management of type 2 diabetes, Rybelsus® (semaglutide). HCPs were overwhelmingly positive towards the new innovation by Novo Nordisk sharing this news over 100 times since its approval, which makes this the most mentioned product in this month’s online HCP conversation around FDA approvals.
The second most mentioned product with 94 mentions was Janssen’s Erleada® (apalutamide) which was approved for the treatment of metastatic prostate cancer. This expansion to the drug’s label was welcomed by HCPs with many sharing the TITAN trial data, showing patient benefit, that was used in the approval submission.
Even though Janssen had the most FDA approvals mentioned by HCPs (three), alongside Merck & Co and Mylan Labs, Novo Nordisk had the highest average number of mentions per approval over the month of September.
| Manufacturer | Approvals | Mentions |
| Janssen | 3 | 201 |
| Novo Nordisk | 1 | 118 |
| Meck & Co (MSD) | 3 | 68 |
We are tracking the HCP reaction to FDA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates within the Tracking section of CREATION Knowledge.


