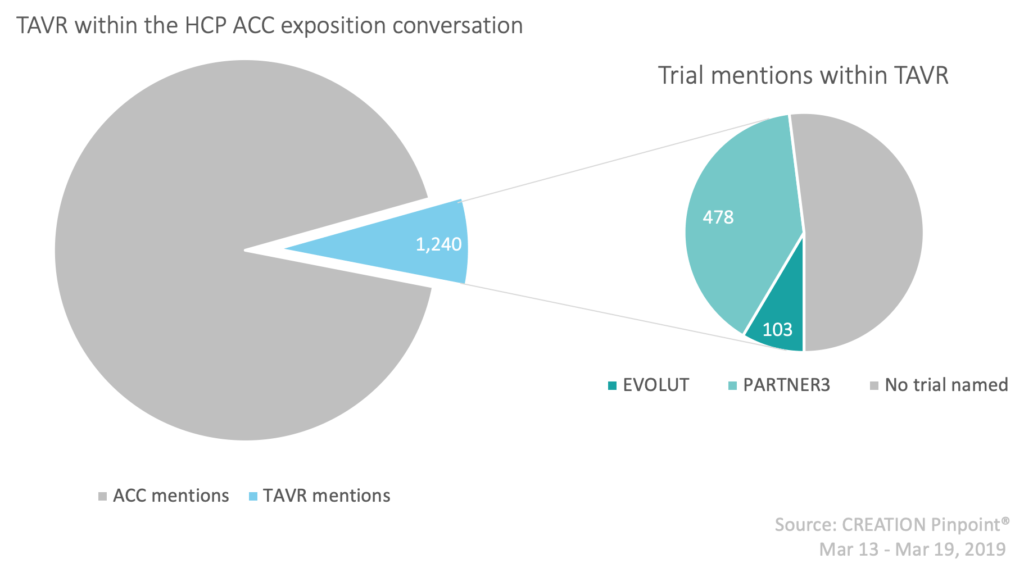
The American College of Cardiology (ACC) annual exposition ran for the 68th time this year from the 16th-18th March 2019, bringing together healthcare professionals (HCPs) in cardiology to hear from leading global cardiovascular professionals about the latest science and innovation in the therapy area. With over 15,000 HCP Twitter posts directly relating to the 2019 ACC exposition from 2,430 unique HCP authors globally, this piece will narrow its focus to one main topic of online HCP discussion from the exposition: transcatheter aortic valve replacement.
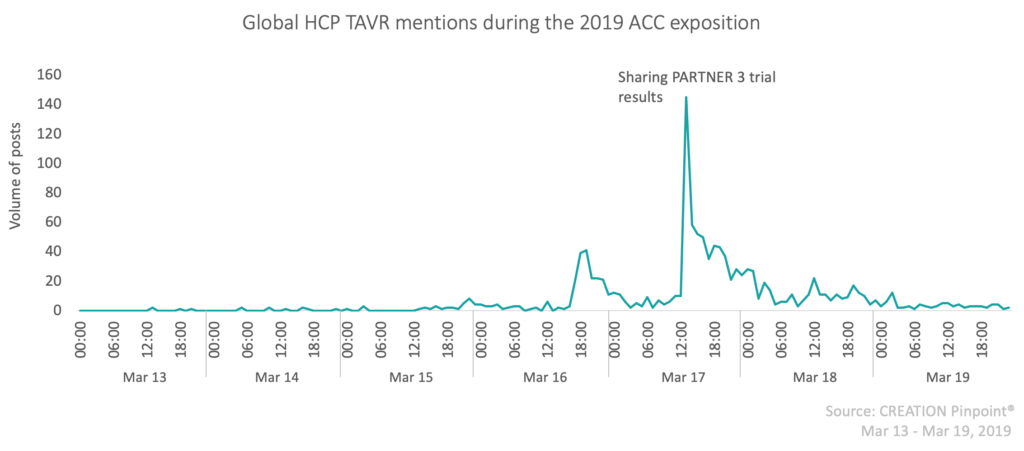
Transcatheter aortic valve replacement (TAVR), also referred to as Transcatheter aortic valve implantation (TAVI) is a “minimally invasive procedure conducted to replace a narrowed aortic valve” using a catheter in patients with aortic stenosis. Currently, this procedure is typically considered an option in patients for whom surgical aortic valve replacement (SAVR) carries an intermediate or high risk of complications. However, promising results were presented at the 2019 ACC exposition from two key trials, PARTNER 3 and EVOLUT, sponsored by Edwards Lifesciences and Medtronic respectively. Both trials sought to evaluate and compare the use of SAVR and TAVR using different valve types among low risk patients with aortic stenosis, and their results may have implications for the prevalence of TAVR in a wider patient population. The ACC described these collectively as “landmark trials”, the results of which “will expand the penetration of TAVR in the treatment of aortic stenosis”. But what do HCPs think?
The online reaction from HCPs regarding the presentation of these trial results was extremely positive, with some even saying they would now be looking for when not to use TAVR, calling for TAVR to be the new standard of care for patients. This is a topic that HCPs seem to view as being extremely important, and their posts give the sense that these results could have a direct impact on practice, and therefore the benefits for patients could be huge.
The top shared source throughout the whole ACC exposition Twitter conversation was the NEJM publication of the PARTNER3 trial procedure and results, with 113 HCP posts sharing a link to the article.
Nicolas Thibodeau, a cardiologist from Québec, started a Twitter poll after the presentation of the trial results, seeking the opinions of other HCPs regarding TAVR as a treatment option in a specific low-risk patient case. Results showed that 73% of voters opted for TAVR over mechanical or tissue SAVR.
HCPs were particularly emotive in their use of language, describing the implications of the trial results as “historic” and “ground-breaking”.
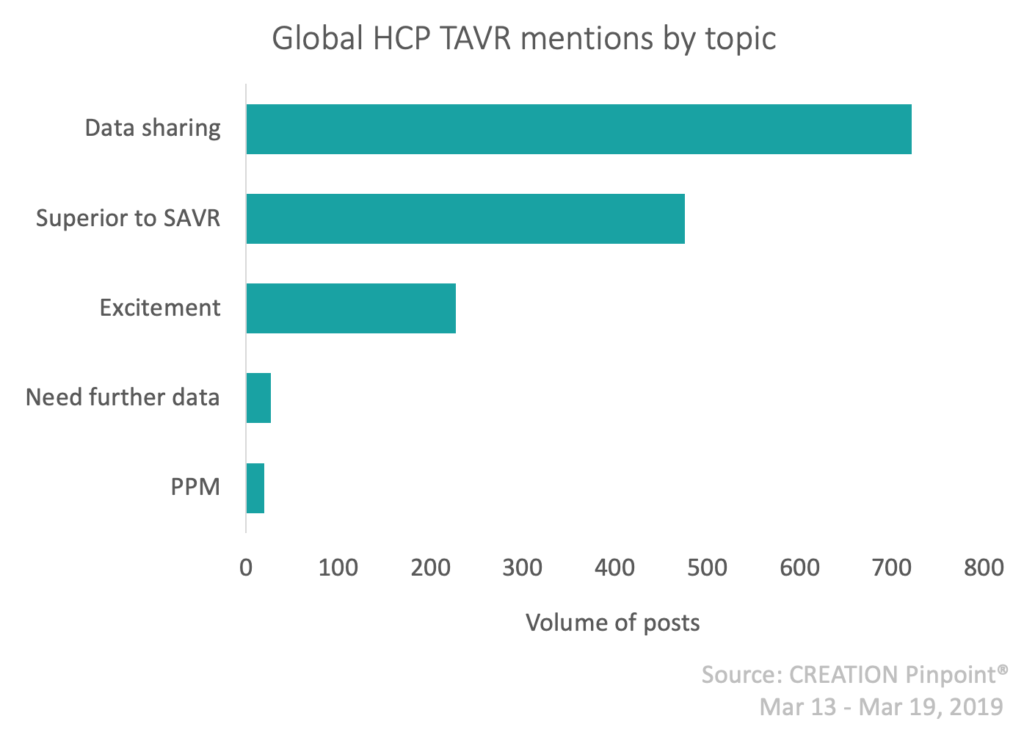
There was however, some acknowledgement of the need for a long-term follow up, (already planned for PARTNER3) but this was somewhat superseded by the immediate success of these trial results.
Comments were also made on the use of only one implant valve type within the trial, perhaps warranting a further trial comparing this to the use of alternative implant valves.
The largest emerging concern though for HCPs is around permanent pacemaker (PPM) implant rates amongst patients who underwent the TAVR procedure during these trials. One Twitter conversation in particular engaged many HCPs in a discussion around this issue. The overarching HCP opinion is that PPMs were not necessary in all patients in whom they were implanted, and there was a call for a better standard of care after the TAVR procedure.
However, some HCPs were so optimistic about the implications of TAVR after these trial results that they brought into question the use of SAVR treatment altogether in the future. Although there is a short list of patient exclusion criteria for TAVR, it is likely to become the commonplace procedure, with the worry for some HCPs being that perhaps the risk will be increased for patients who do then undergo SAVR, given the expected low frequency of surgery performances and potential challenges for surgeons to maintain competency.
In light of the recent trial results presented at the 2019 ACC exposition, it is clear that HCPs consider TAVR to pose an exciting opportunity in the development of aortic stenosis treatment. With the SAPIEN 3 valve used in the PARTNER 3 trial expected to receive approval from the Food and Drug Association and European Medicines Agency towards the end of this year, the expanded use of TAVR could perceivably become a key moment in the history of cardiology.
For Edwards Lifesciences and Medtronic, tracking the online behaviours and perspectives of HCPs in this way, in order to understand the needs and interests of their customers could prove helpful with the market launch of these two new valves. In our previous congress article with a focus on ASCO and ESMO, specific ways of leveraging HCP participation at congresses were identified which, if implemented by pharmaceutical companies, could help to generate HCP advocates and thus impact the traction of future trial data.
CREATION Pinpoint was used to monitor the online activity of validated HCPs on Twitter during a window of time surrounding the ACC exposition (13th-19th March 2019), thus enabling the analysis of HCP engagement within the cardiology space during this time.
The PARTNER 3 trial conducted the TAVR procedure using a balloon-expandable valve, and the EVOLUT trial using a self-expandable valve. Results of the PARTNER 3 trial show that in this cohort of low-risk patients, TAVR was superior to SAVR in the reduction of severe side-effects including death, stroke and rehospitalization one year after the procedure. Similarly, results of the EVOLUT trial showed that TAVR was noninferior to SAVR for death and disabling stroke 24 months after the procedure.
 By Laura Marsh
By Laura Marsh