Janus kinase (JAK) inhibitors, a treatment option developed in the past decade for immune-mediated diseases are currently being tested beyond their currently marketed indications for rheumatoid arthritis and psoriatic arthritis. The role of JAK inhibitors in treating inflammatory conditions is yet to be defined and online unprompted HCPs conversation can provide a glimpse into its emerging landscape.
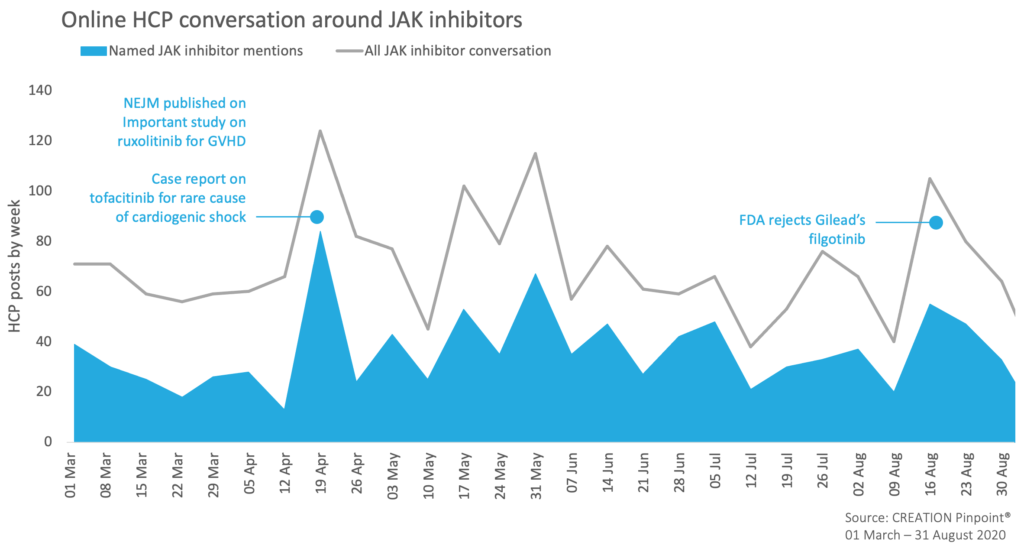
As a drug class, JAKs were discussed by HCPs globally in more than 1,800 Twitter posts between March 1st and August 31st, 2020. HCPs preferred to engage in conversation around data publications, congress presentations and regulatory decisions. The dynamic online conversations of HCPs spiked around these topics with more than 51% of their posts referring to named JAK inhibitor therapies. Here are some of the learnings from their online professional conversations.
HCPs actively keep track of new developments of JAK inhibitors by engaging with and sharing trial data
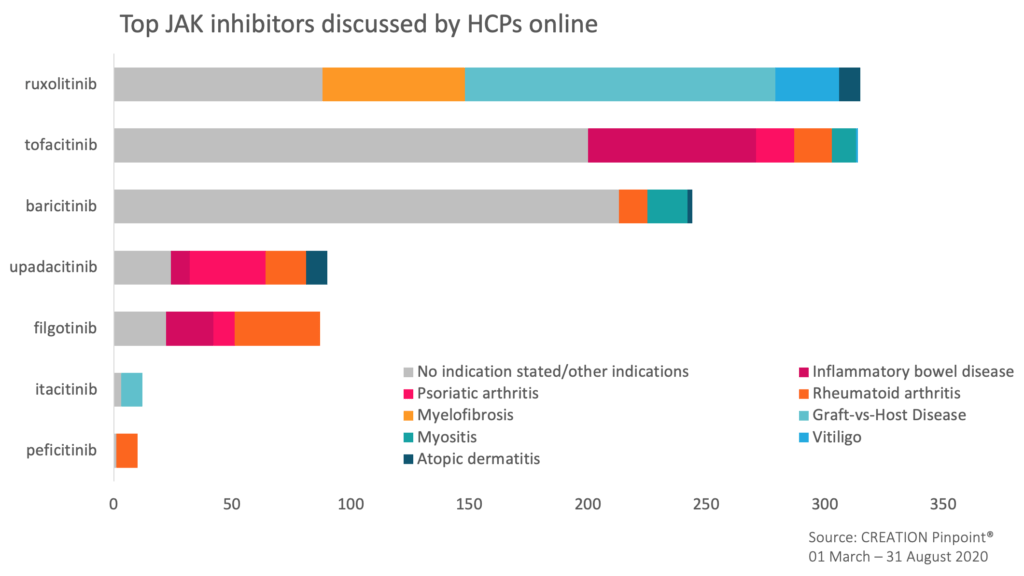
Each of the mentioned JAK inhibitors was discussed in the context of a variety of therapy areas and indications and revealed the focus of HCP interest.
In April, ruxolitinib received significant HCP attention online in a Graft-Versus-Host Disease (GvHD) setting. HCPs shared various positive ruxolitinib data publications and content from this year’s European Bone Marrow Transplant (EBMT) virtual conference relating to REACH2 trial findings and defining the ruxolitinib-refractory patient population in order to support the sequencing of therapies in patients with GvHD.
To me this is a big deal💥: REACH-2 met it’s primary endpoint (RCT of ruxolitinib vs BAT for steroid-refractory acute #GVHD)https://t.co/Dt0i4Jdwh6
Are the REACH-2 results practice changing?
Had the practice already changed?
Should ruxo be the control arm for future studies? 🤔 pic.twitter.com/UxeeLR252o— Jordan Gauthier (@drjgauthier) May 9, 2020
The product was also discussed in relation to its efficacy in myelofibrosis setting, with HCPs sharing study data and patient stories.
With recent data being released in inflammatory bowel disease (IBD), tofacitinib, also known by the brand name Xeljanz, was talked about in the context of this indication the most. HCPs highlighted the latest positive data for its use in treating ulcerative colitis. The review published in the UEG Journal aimed to understand its potentially “game changing” application in UC treatment and was reposted by HCPs online. HCPs also celebrated successful use of this treatment in PsA resharing posts by their peers.
HCPs are optimistic about trial data but put emphasis on product safety
When discussing JAK inhibitor trial and study data, the topic of safety emerged as a key area of interest for HCPs, making up 26% of the scientific data conversation.
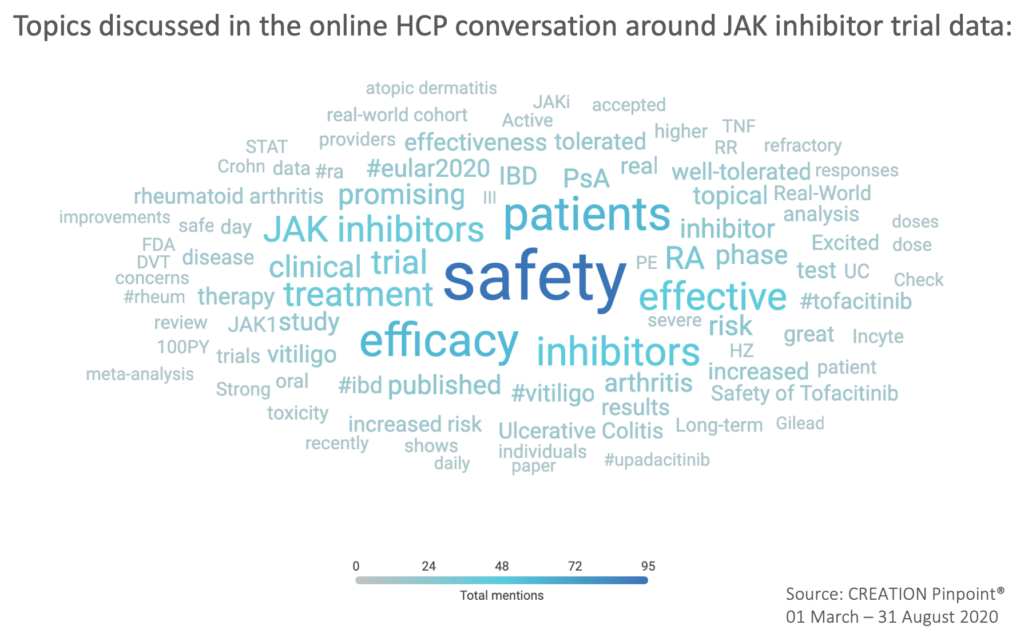
HCPs drew attention to tofacitinib safety data in Ulcerative Colitis published in Clinical Gastroenterology and Hepatology and in Crohn’s disease, presented at Digestive Disease Week (DDW) 2020.
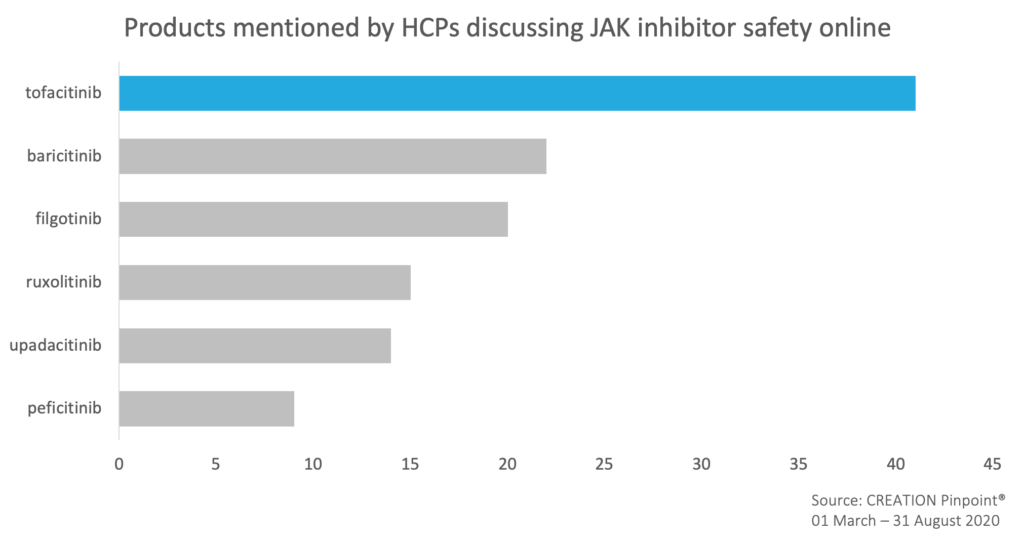
Following the ACTT-2 trial results for a baricitinib and remdesivir combination to treat coronavirus, a UK safety reminder for use of baricitinib in patients with diverticular disease and taking steroids or NSAIDs was shared by HCPs online.
JAK inhibitors’ safety profiles continued stirring HCP online dialogue with an unexpected FDA rejection of Gilead’s filgotinib over toxicity concerns. While sharing the news, HCPs wondered how much of a setback this would be and expressed hope for the upcoming additional data.
Filgotinib Decision Delayed by FDA. Instead of being the next approved RA drug, the FDA issued a complete response letter; citing concerns over sperm studies and the risk/benefit behind the 200 mg dose. Delay till 2021? 2022? https://t.co/6MHiGtOxCM
— Dr. John Cush (@RheumNow) August 19, 2020
Shocked that FDA rejects @Gilead #filgotinib over toxicity concerns but important to characterize effect on sperm through MANTA AND MANTA-RAY studies, hope for results soon! #RA https://t.co/VHQVagY2f5
— Ara Dikranian (@RumorDoc) August 19, 2020
How to support HCPs in the JAK environment
The online HCP JAK inhibitor conversation is currently driven by data with a clear focus on product safety.
By supporting and engaging HCPs online in the areas they are already interested in, pharmaceutical companies can develop a leading voice in the space. Understanding the landscape and HCP views towards emerging treatments and identifying areas to support professionals on issues they care about could give HCPs more confidence in the novel treatments in the online and offline space.
Get in touch to find out more about ways to keep a finger on the pulse of the changing landscape to inform your engagement and launch plans.
Methodology:
- The online unprompted HCP conversation on Twitter in English language, occurring between the 1st March 2020 and 31st August 2020 was analysed using CREATION Pinpoint, the world first database of confirmed HCPs online, tracking over 2 million HCPs worldwide.

 By Laura McIntyre
By Laura McIntyre