Discover what healthcare professionals (HCPs) think about pharmaceutical products and their manufacturers, as it happens, through CREATION.co’s tracking updates. How are HCPs responding to the latest trial results for COVID-19 vaccine candidates? How are HCPs talking about or engaging with the Top 50 pharma companies on social media? Each week CREATION.co’s tracking updates bring you the latest insights from the conversation of HCPs across the globe discussing these topics and more.
September has seen a dramatic increase in the online healthcare professional (HCP) conversation around COVID-19 vaccine candidates. The number of HCP authored posts and the number of HCP authors participating in the dialogue have more than doubled compared with August.
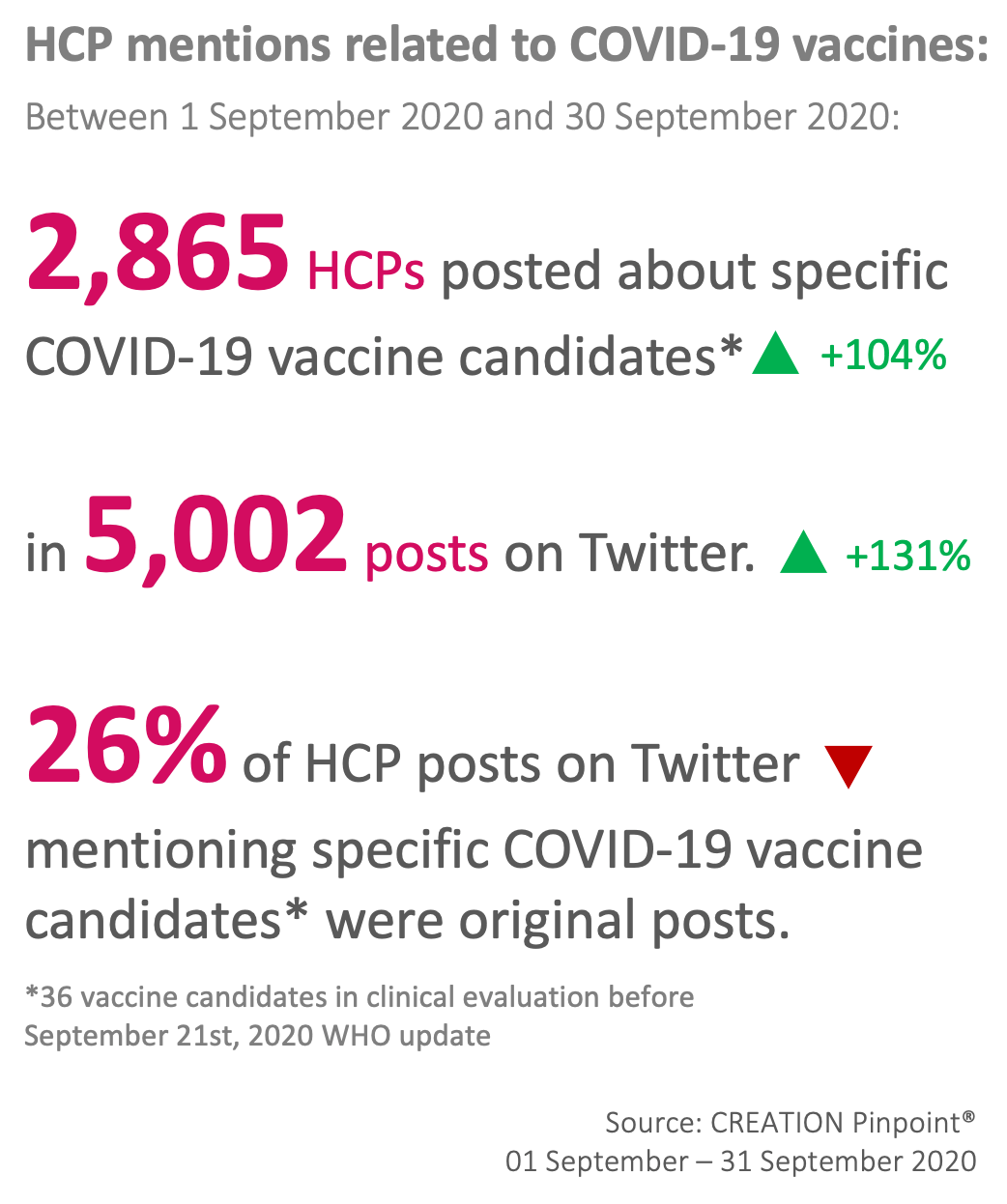
The Top 10 covid-19 vaccine candidates mentioned by HCPs on Twitter in September
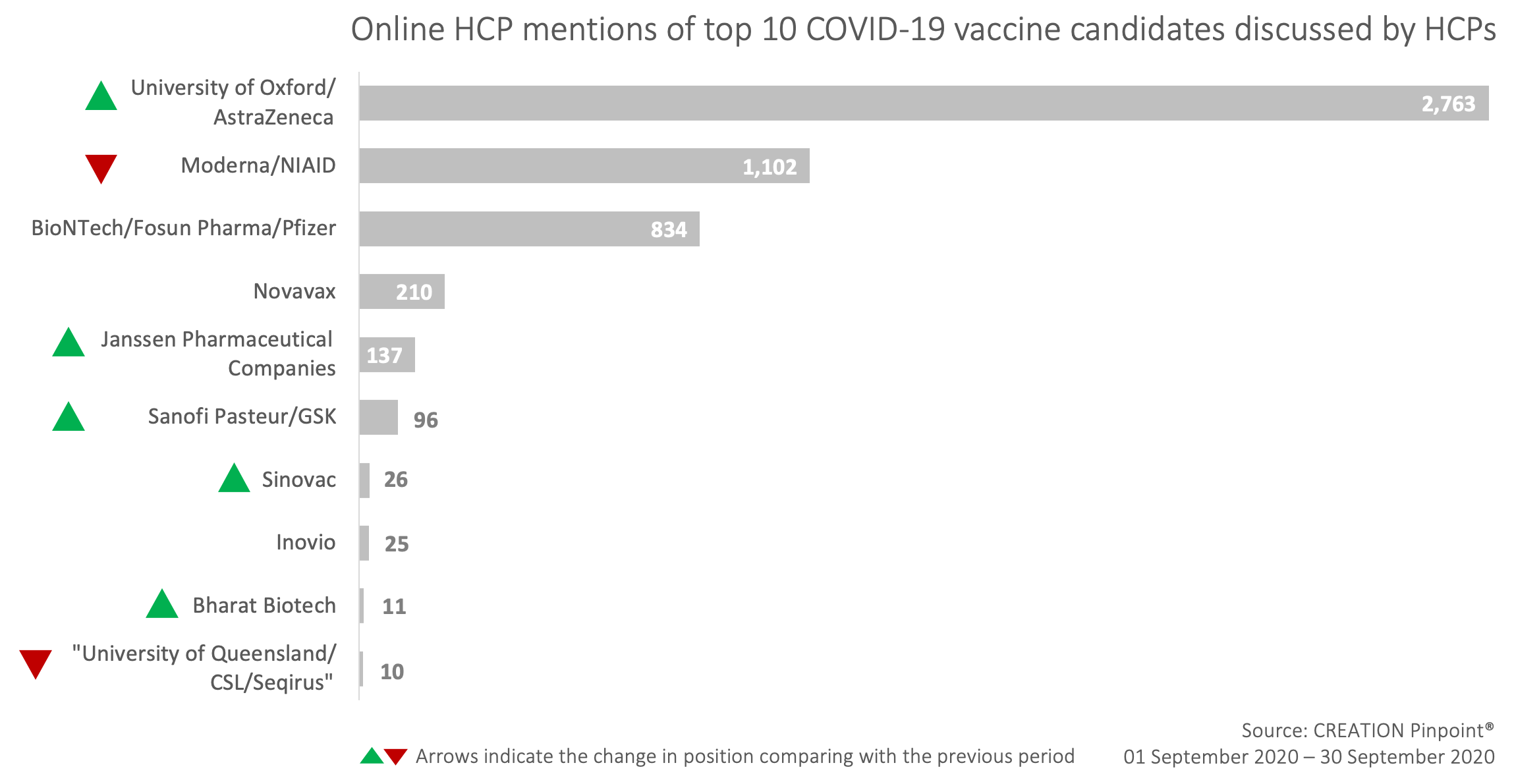
September’s insights from HCPs mentioning covid-19 vaccine candidates on Twitter
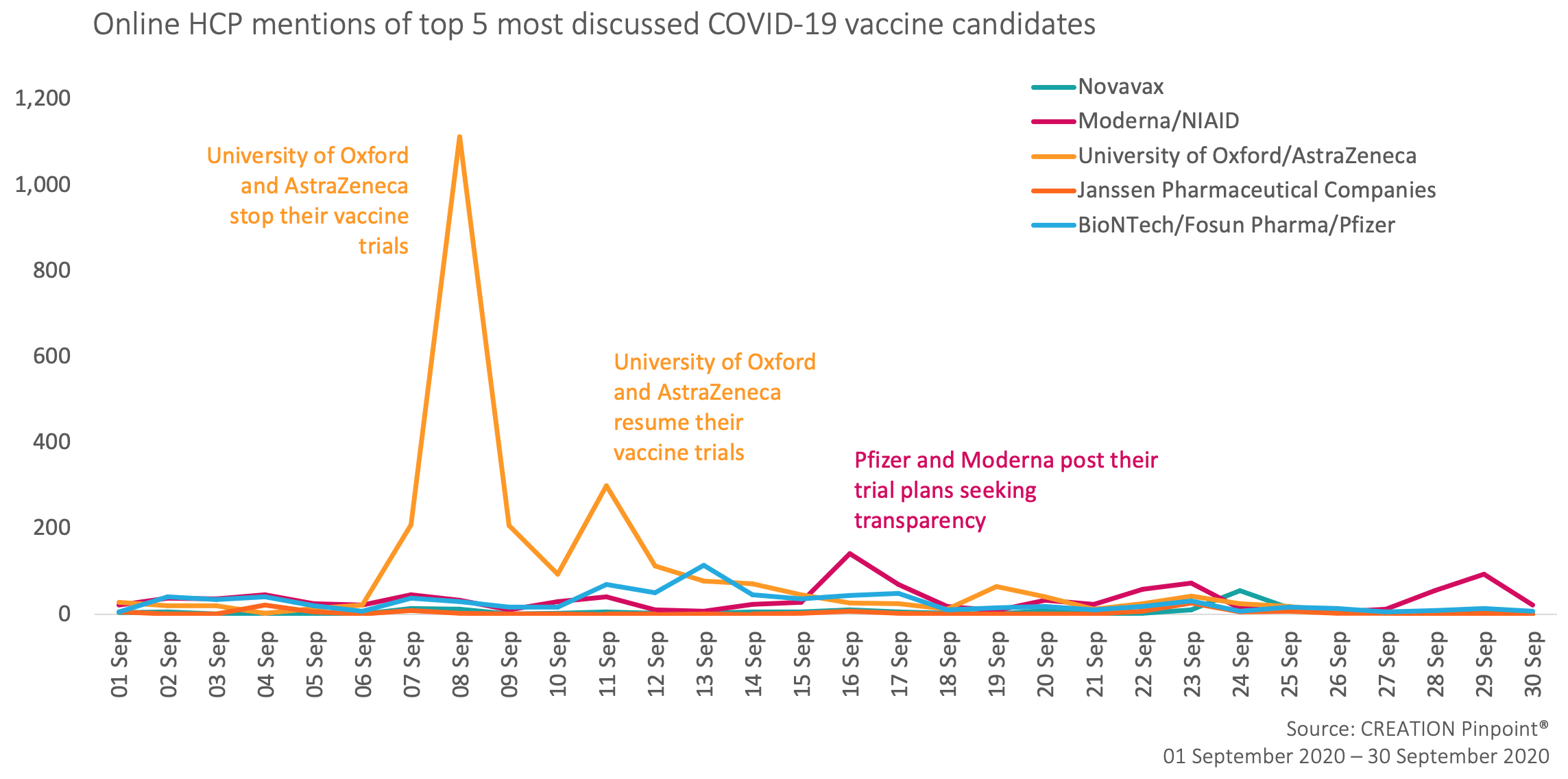
In September, HCPs were more inclined to repost and retweet content relating to the COVID-19 vaccine candidates rather than produce original posts. This could be related to the nature of the key online HCP conversation driver in this period – the news about AstraZeneca pausing their vaccine trial due to safety concerns. On the 8 September AstraZeneca and Oxford University announced that the trials have been “voluntarily paused” in order to review and investigate a serious adverse event that had occurred among the trial volunteers. The news was shared by HCPs all around the world curating nearly 1,500 posts on Twitter alone and also appeared in our monthly Top 50 Pharma tracker.
SAFETY FIRST
While the majority of HCPs merely shared the news, a considerable portion of HCPs expressed a positive opinion toward the AstraZeneca action, saying that while this could have been a “false alarm” , it is a “good sign” and means that the Phase 3 trial is being carried out properly with an “appropriate focus on safety”. Some HCPs, such as GP Sarah Jarvis, defended the setback, stating that contrary to “antivaxer” claims, this is the proof of how seriously researchers are taking the safety of patients.
Other HCPs including an infectious diseases doctor in the US used social media to clarify what this means for the future of the vaccine and tried to put this into perspective, highlighting that this is normal practice when it comes to the clinical trials.
An epidemiologist in India spoke on a podcast, in which he explained “why Oxford-AstraZeneca pausing COVID vaccine trial is a good sign“.
Many HCPs responded to the news highlighting the importance of the clinical trials and were positive towards the manufacturers signing a pledge not to cut corners in safety when seeking the FDA approvals for their candidates.
The vaccine trial was soon announced to be resumed after reviewing the safety data and HCPs shared their excitement about the good news online, anticipating the Phase 3 trial results. Some HCPs expressed the probability of the trial being paused again, as safe “vaccines take time”.
This story caused AstraZeneca’s and Oxford University’s vaccine to claim back the position of most discussed candidate, leaving others far behind.
TRANSPARENCY INCREASES TRUST
Another key event that drew HCP attention online was Moderna’s decision to post its vaccine trial plans in pursuit of greater transparency. Their efforts were met with HCP positivity saying that transparency can increase trust in vaccines in general and guiding their followers to manufacturer’s resources. Some HCPs shared other authors’ posts that commended Moderna’s initiative and encouraged other manufacturers to step up.
SO WHAT?
HCP response to last month’s event signifies that greater transparency and accountability leads to greater trust in Pharma. HCPs use their social media platforms to keep their public and peer audience up to date with trusted information seeking to bring clarity. The pharmaceutical industry therefore has the opportunity to use this time to build meaningful relationships with its HCP customers and create a reputation of a trusted partner.
We will be tracking the online HCP conversation to identify trends and change in views relating to COVID-19 vaccines. You can stay up to date with HCP insights by subscribing to CREATION Knowledge e-journal.
- Data for this research was analysed using CREATION Pinpoint® from the online Twitter conversations of HCPs around the world in English language (other languages are available), between September 1st – September 30th, 2020.
- Vaccines tracked were the 36 COVID-19 vaccine candidates in clinical evaluation before 21st September 2020 (WHO Draft landscape of COVID-19 candidate vaccines).
 By Laura McIntyre
By Laura McIntyre 
