HCPs show excitement about new EMA drug approvals
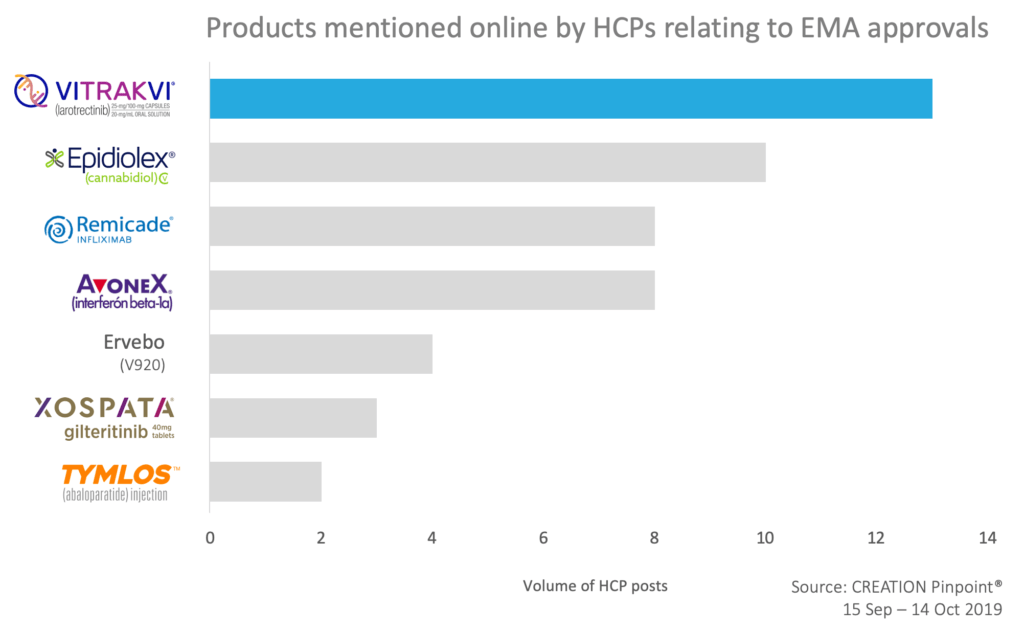
In the EMA online conversation, related to newly approved products, Bayer’s Vitrakvi received a lot of positive engagement from HCPs as the “revolutionary” tumour agnostic therapy for TRK fusion cancer.
Not far behind was Greenwich Biosciences’ Epidiolex (cannabidiol). HCPs excitedly shared the good news but agreed that the “hurdles remain before the drug becomes available on the NHS “ as BMJ states.
Among other approvals discussed by HCPs online was the recommendation for approval of subcutaneous formulation of infliximab and expectations for the interferon beta approved use during pregnancy and breastfeeding.
Concerns over EMA approval standards
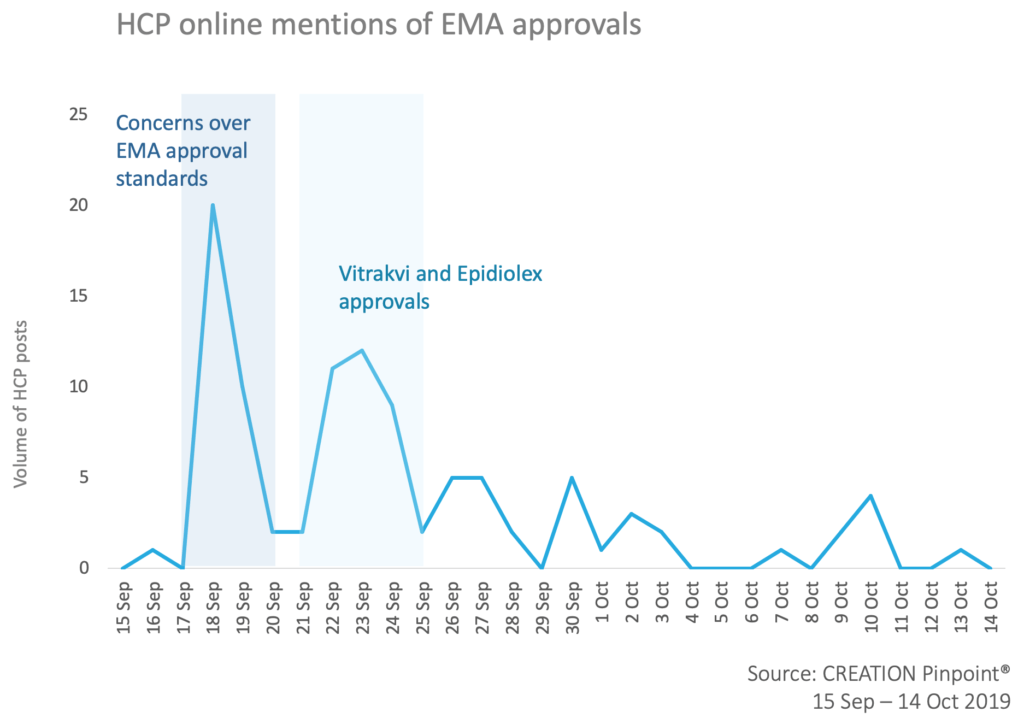
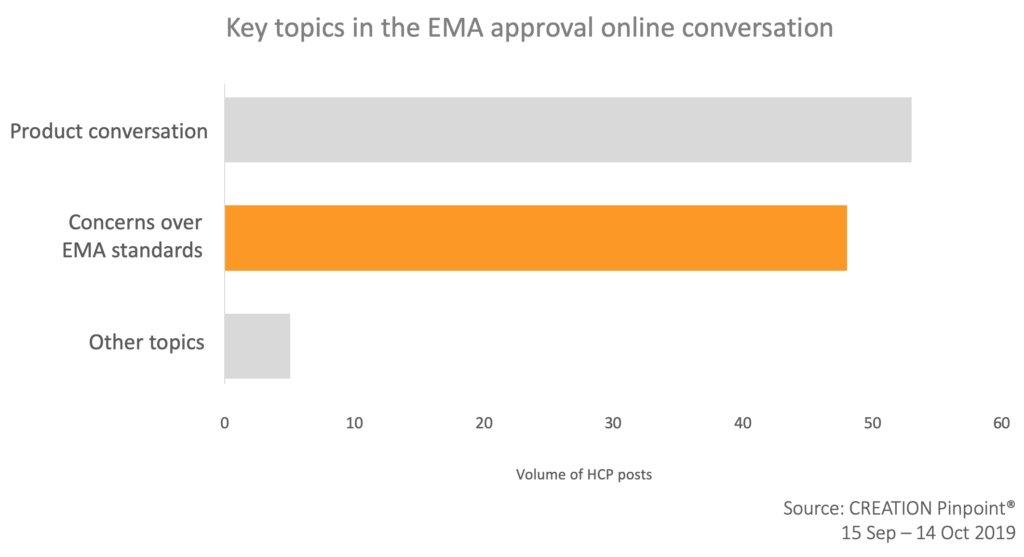
A large proportion of the online conversation related to concerns about EMA approval standards for oncology. A study by BMJ “Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross-sectional analysis” published on the 18th of September caused a spike in the online HCP conversation. HCPs shared the link to the article quoting that the new study “raises questions about the therapeutic benefits & economic value of new cancer drugs approved by EMA between 2014-16.”
An oncologist from Pakistan suggested that the FDA and EMA should “now adopt the clinical benefit scale by @myESMO or @ASCO”.
A video abstract was also shared by several HCPs illustrating that 50% of randomised clinical trials supporting EMA cancer drug approvals had a high risk of bias.
We are tracking the HCP reaction to EMA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates, including FDA drug approvals, within the Tracking section of CREATION Knowledge.
Read last month’s EMA Approval Tracker:
Methodology notes:
- Data for this research was analysed from the online Twitter conversations of HCPs talking about EMA approvals in English language (all other languages are available), between September 15th and October 14th 2019.
- Between 15th September and 14th October 98 HCPs posted about EMA approvals 106 times all over the world.

 By Laura McIntyre
By Laura McIntyre