Every month CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
The conversation Level of HCPs discussing product launches on Twitter
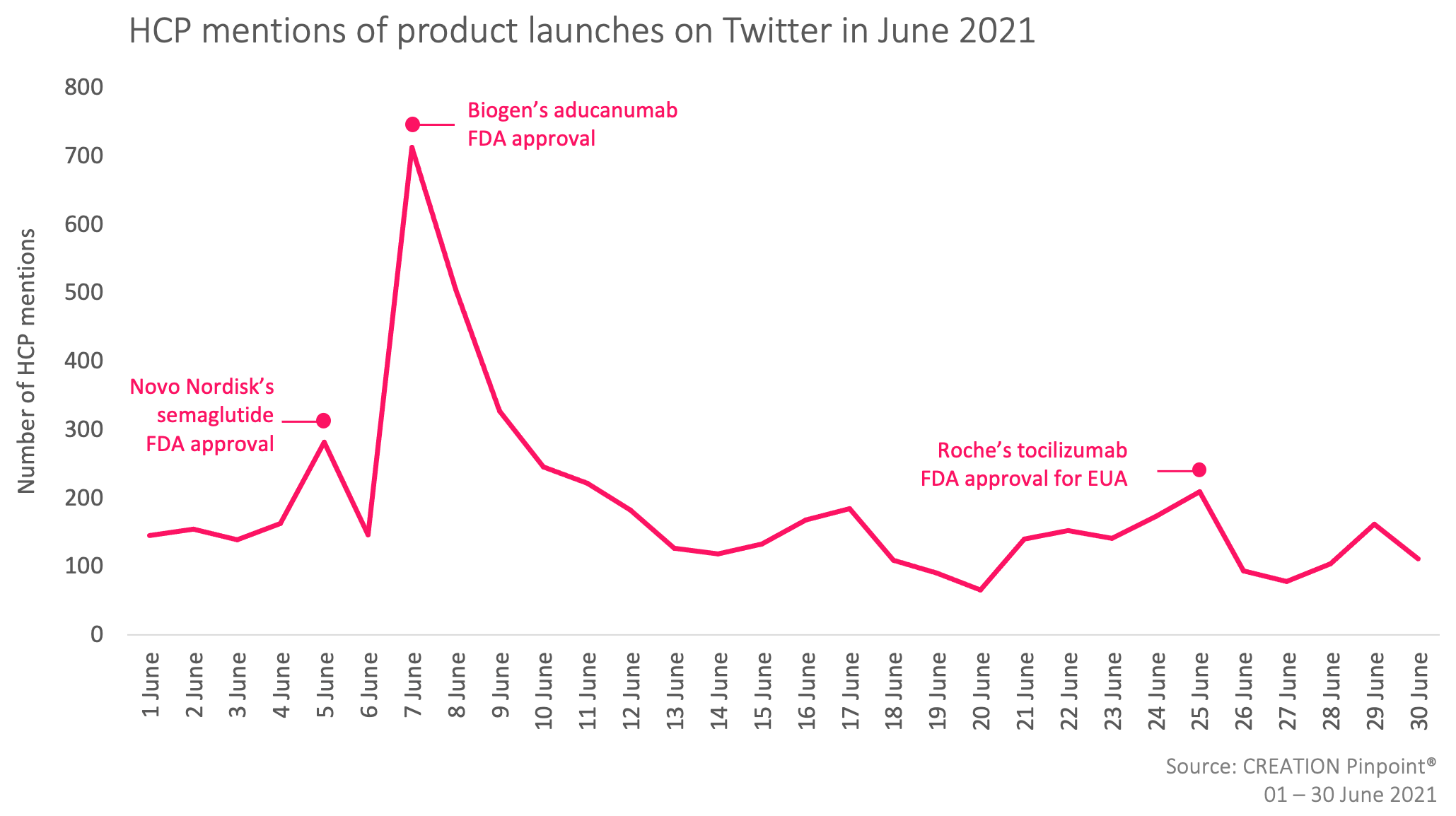
Throughout June 2021 we tracked the global conversations of over 3,000 HCPs who posted more than 5,500 English-language Twitter posts.
The latest product launch insights from
HCPs online
In June 2021, the new product approvals that caught HCPs’ attention were Biogen’s Aduhelm (aducanumab) Alzhiemer’s treatment, Novo Nordisk’s Ozempic or Wegovy (semaglutide) type 2 diabetes and chronic weight management treatment, and Roche’s Actemra (tocilizumab) COVID-19 treatment for Emergency Use Authorisation (EUA).
HCPs shared the announcements of the new product approvals. Many HCPs, such as Clinical Neurologist, Dr Alex Knopman, Geriatrician, Gary Blanchard, and Psychologist, Dr. Tracey L. Smith shared their scepticism towards the FDA’s decision to approve Biogen’s Aduhelm, many of which discussed the drug’s said lack of efficacy and “failed trial”.
FDA approves first new Alzheimer’s drug in almost 20 years. This is huge news – but read the article. The road to aducanumab being approved has been rocky. https://t.co/aNTl5IcFhW
— Dr Alex Knopman (@focusneuro) June 7, 2021
Dark day for FDA. Approving a drug on the basis of post-hoc analysis of a failed trial, in the context of a 2nd failed trial, a worrisome safety signal, and an exorbitant price. Promoting false hope for Alzheimer’s is not in the best interest of patients https://t.co/b58Kn5QjW9
— Joseph Ross (@jsross119) June 7, 2021
Several HCPs positively discussed Novo Nordisk’s Ozempic FDA approval and the drug’s weight management ability, especially for diabetic and obese patients. Some expressed hopeful anticipation towards the drug’s possible future approval in Europe. HCPs also shared the announcement of Roche’s Actemra COVID-19 treatment’s FDA EUA approval. Although only approved for emergency use in critical care and hospitalised children and adults receiving systemic corticosteroids and requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or ECMO; HCPs shared the approval positively as “good news for patients”.
Exciting times in Bariatric Medicine – hopefully we will soon have this as an option for people living with Obesity in Europe too! #supportnotstigma #livingwithobesity
U.S. FDA approves Novo Nordisk's semaglutide as obesity treatment https://t.co/8N9z5LJTgJ
— Michael Crotty (@DrMCrotty) June 5, 2021
RECOVERY Impact: Tocilizumab
@US_FDA grants Emergency Use Authorisation for hospitalised patients with COVID-19 who are receiving oxygen (+/-ventilation) & corticosteroids
Good news for patients
Thanks to all involved
Clinical trials save lives https://t.co/JNFmpqFGgy
— Martin Landray (@MartinLandray) June 25, 2021
The three most shared links from HCPs discussing product launches in June were:
- An FDA article about their decision to approve Biogen’s Alzheimer’s treatment, using the Accelerated Approval pathway.
- An FDA article about their approval of Wegovy, which is now the first FDA-approved treatment for chronic weight management since 2014.
- A New York Times article about the FDA’s aducanumab approval for Alzheimer’s disease“ despite fierce debate over whether it works”.
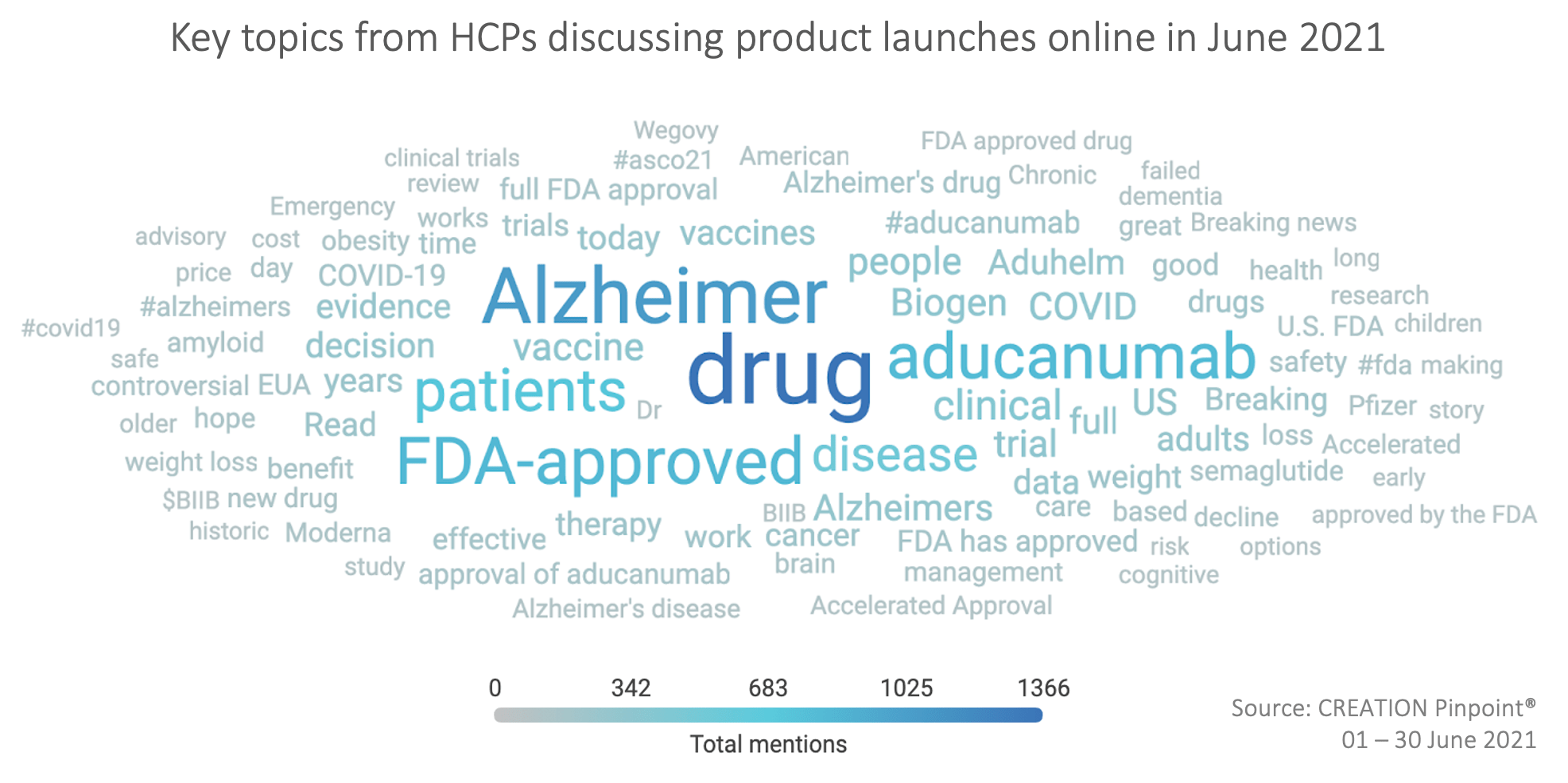
Throughout the month, aducanumab’s FDA approval provoked a high volume of discourse about Alzheimer’s disease, with HCPs mentioning the illness over 1,300 times in their online discussions.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 June and 30 June 2021 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.Between 01 June and 30 June 2021, there were 5,596 HCP mentions of new pharmaceutical product launches and drug approvals from 3,471 unique HCP authors from around the world.
- Between 21 May and 20 June 2021, there were 3,256 UK HCP mentions of respiratory disease and related terms, from 1,524 unique UK HCP authors.

