Respiratory syncytial virus (RSV) is a common respiratory virus that usually causes mild, cold-like symptoms. While most people recover within a couple of weeks, RSV can be particularly serious for infants and older adults. The virus is not new but has been surging in the past month. There are many thoughts as to why RSV cases are particularly high this year, but experts agree that the lifting of COVID-19 precautions have left many vulnerable to infection. On Mastodon, Infectious Disease Professor, Carlos E. Figueroa Castro, explained that RSV cases have added to the burden that COVID-19 and flu has placed on hospital systems.
There is currently no approved vaccine to prevent RSV, but top players in COVID-19 vaccination and treatment, Pfizer, Moderna, Johnson & Johnson and GSK are each investing in RSV vaccines.
HCPs have not missed this news, and alongside their clinical experience of rising cases, have been beginning to take notice of the companies’ pre-launch activities.
Why is an RSV vaccine needed now?
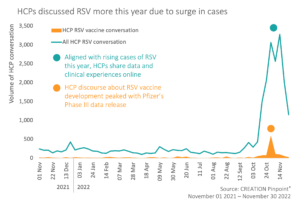
Even though only 7% of the overall RSV online HCP conversation over the past year has mentioned vaccine development, in the week of October 31st, data updates and press releases have piqued the interest of HCPs online. With the news of vaccines on the horizon, HCPs are building hope to protect the vulnerable, reduce health system burden and save lives.
Hope on the horizon!
The race to make vaccines for Respiratory Syncytial Virus (RSV)
Millions of people a year are
After decades of failure, four vaccines, GSK, Janssen, Moderna and Pfizer, are now in late-stage trials #IDTwitter #MedEd #TwitteRx https://t.co/G42SWkbnT8— Antibiotic Steward Bassam Ghanem 🅱️C🆔🅿️🌟 (@ABsteward) December 13, 2021
With this year’s powerful RSV surge adding to the burden on healthcare systems, HCPs have been calling for more research into vaccine development to prevent future cases. Paediatric hospitalist, Andrea Hadley, specifically called out the impact of the virus on children, pushing for an approved vaccine to ‘do better’ for them.
RSV overwhelming children’s hospitals around the country. Need research, prevention, treatment. If a new disease #COVID can have vaccine, treatments w/in 2 yrs, we NEED to do better for kids! Push RSV vaccine to the finish line! https://t.co/CDJfjouuKZ
— Andrea Hadley (@AndreaHadleyMD) October 25, 2022
Further, expanding hospital capacity is only a short term solution, according to epidemiologist Jennifer Nuzzo, who said vaccines are needed as a matter of urgency.
This is a situation that requires our urgent attention. In the short term we need to help these hospitals expand their capacity. We also need to accelerate progress on RSV vaccine development. https://t.co/GuT48J7hX5
— Jennifer Nuzzo, DrPH (@JenniferNuzzo) October 21, 2022
Who’s in the RSV vaccine race?
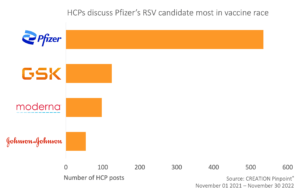
HCPs are talking about each of the RSV vaccines in development, but Pfizer, with their latest positive efficacy data has gained the most traction online.
While in the pre-approval and launch phase, these companies have begun above-brand marketing, broadly raising awareness of the virus and implications, as well as publishing their data.
Currently, there is no vaccine for respiratory syncytial virus or #RSV, which is a common cause of serious respiratory illness. We are using revolutionary tools to develop a potential RSV vaccine.
— Pfizer Inc. (@pfizer) December 6, 2021
#DYK RSV causes around the same number of hospitalisations in older adults as seasonal flu each year?
However, advances in science mean we could be on the cusp of finding new solutions.
— GSK (@GSK) October 20, 2022
Could you recognise the symptoms of #RSV? It can be easily confused with flu or other respiratory infections and can be severe in older adults. This #RSVAwarenessMonth, it's time to get to know RSV. If you have flu-like symptoms, consider RSV and speak to your pharmacist. pic.twitter.com/wHJePDKgTR
— Janssen UK (@JanssenUK) October 20, 2022
Each of these official account posts have received engagement from HCPs and other members of the public, adding to the online discourse bringing awareness to RSV.
During ID Week, GSK collaborated with a KOL online, to present their latest RSV vaccine trial data. The session was a success, with “standing room only” according to Nicole Theodoropoulos, showing the level of interest in the research.
https://twitter.com/nikkitheomd/status/1583166320706654213
Pfizer’s press release sharing top-line results of their Phase 3 maternal immunisation trial was disseminated by various HCPs who were excited to see positive data and looking forward to more. Ahead of the publication of peer-reviewed articles, HCPs do not yet have sufficient evidence to be fully convinced by the data, but are positively anticipating the full trial results.
A new RSV vaccine has high efficacy VS severe infection in infants in this Phase 3 clinical trial.🙂
RSV can cause severe illness in young children & the elderly. A vaccine is most welcome.
Looking forward to reading peer-reviewed paper.
Press Release: https://t.co/0apTskXkKg pic.twitter.com/YKLuVvkRCf
— Isaac Bogoch (@BogochIsaac) November 1, 2022
Subunit protein RSV maternal vaccine completed phase 3 trials – data not available but expect promising results. Maternal antibody crosses the placenta to protect young infants. RSV hospitalises -6000 young children, mostly infants, each year in Australia https://t.co/pIjY6R6nJb
— Fiona Russell (@Fiona_M_Russell) February 16, 2022
The significance of even the available data is not amiss to HCPs. Without any currently approved vaccine globally, the possibility of an option for as soon as next year’s RSV season is big news.
The is HUGE!!
There are currently no RSV vaccines approved anywhere in the world. Regulatory submissions based on the phase III data are anticipated in the second half of 2022 #IDTwitter
Data from PR sooner #IDWeek2022 https://t.co/IQ1URs3HgP pic.twitter.com/OZzVTQj87Z— Antibiotic Steward Bassam Ghanem 🅱️C🆔🅿️🌟 (@ABsteward) October 13, 2022
What Do HCPs Think?
HCPs are inspired by the early data, aware that a successful candidate would have a huge impact on the global healthcare system. Infectious disease specialist, Dr Neuro, labelled Pfizer’s vaccine data as “huge news for the babies” and physician Alok Patel said an effective RSV vaccine would be “amazing” for those working in overcrowded hospitals.
Huge news for the babies!
Pfizer’s bivalent RSV vaccine has demonstrated amazing efficacy (81.8% against severe lower respiratory illness). This was well tolerated and with results meeting criteria, Pfizer has stopped enrollment and will plan to submit by end of 2022! pic.twitter.com/dObCKEjBJT
— Dr. Neuro 🏳️🌈 🦠💉💪 (@Neurofourier) November 1, 2022
To all my fellow pediatricians and subspecialists rolling through overcrowded ERs and hospitals, hang tough.
Also, how amazing would an effective RSV vaccine be?!?!— Alok Patel (@AlokPatelMD) October 21, 2022
But online HCPs are eager to see the full data, and some have reminded their peers not to get ahead of themselves with the limited data currently available. In mainstream media, Moderna was called out for promoting their product before releasing full safety data, and infectious disease physician, Isaac Bogoch warned about the need for transparent data and appropriate regulatory processes.
"Controversial decision to promote shot before clinical trials are done raises concerns."
It sure does.
Vaccines for RSV are sorely needed, but that means transparency with efficacy & safety data, & going through appropriate regulatory channels.
By @adamsmiller 👇 https://t.co/1DowjKq1no
— Isaac Bogoch (@BogochIsaac) November 12, 2022
Radiologist David Jacobs added his thoughts to this conversation, mindful of the need to maintain public trust, and imploring the medical community to remember that there are no shortcuts where children are involved.
The medical community cannot skip steps in clinical trials and hope to maintain public trust.
There is a process and it must be rigorous especially when children are involved.#RSV #Vaccines #COVID19 https://t.co/NWk3RSNFPG
— David Jacobs (@DrJacobsRad) November 14, 2022
Further, frequent commentator on the latest clinical trial data, Vinay Prasad, put out a plea to his network to seek all available data on RSV vaccine development, calling the press releases “thread bare”.
As part of my series: I have to do the FDA's job for them. Could followers please link to all the data they've seen about the new RSV vaccines. I've yet only seen press releases. Thread bare. Pls link. Tnx.
— Vinay Prasad MD MPH (@VPrasadMDMPH) November 1, 2022
Where next?
The online conversation around RSV vaccine development shows that positive early data and regulatory nods encourage HCPs, while they are eager to see the full results. Before they recommend these vaccines, HCPs need to trust not only their efficacy but just as importantly their safety. While the need for an RSV vaccine is great, the industry needs to be seen to respect the process, and build HCP and public confidence. One way of creating trust with HCPs is to engage in two-way relationships, to understand the need of the HCP and to work alongside Digital Opinion Leaders to communicate and unpack the data for a wider audience as and when it becomes available.
To continue learning about the changing landscape of online HCP conversations and Digital Opinion Leader activation, feel free to reach out to [email protected].
 By Mary Kangley
By Mary Kangley 

