Drug development, safety and approval is underpinned by a thorough and multi-faceted clinical trial process. While a field of its own, clinical trial design and recruitment can present a number of barriers to be overcome to produce sufficient data to fulfil regulatory body requirements. Many considerations have to be made, including optimising trial set-up for ease of patient participation, equitable recruitment requirements to challenge existing health disparities, and adequate design to provide rigorous data to instil confidence in prescribing physicians.
Not only is social media a platform to disseminate clinical trial results, especially during congress meetings when the data is published, but it can also be utilised to identify topics of interest for future research and as a vessel to aid recruitment and avoid under-recruitment. There is evidence that proves authors who tweet about their research can increase publicity and citations for their work, as well as to prove that social media can enhance clinical trial recruitment.
CREATION Pinpoint® has a database of over 3 million HCP online profiles (across platforms) and as such can be used to discover what HCPs think about clinical trial recruitment, as well as to explore behaviours they already deploy to contribute to their research. Over the past 12 months (July 2021-July 2022), CREATION Pinpoint® identified over 6,000 HCP authored posts on the topic of clinical trial recruitment in English language globally on Twitter.
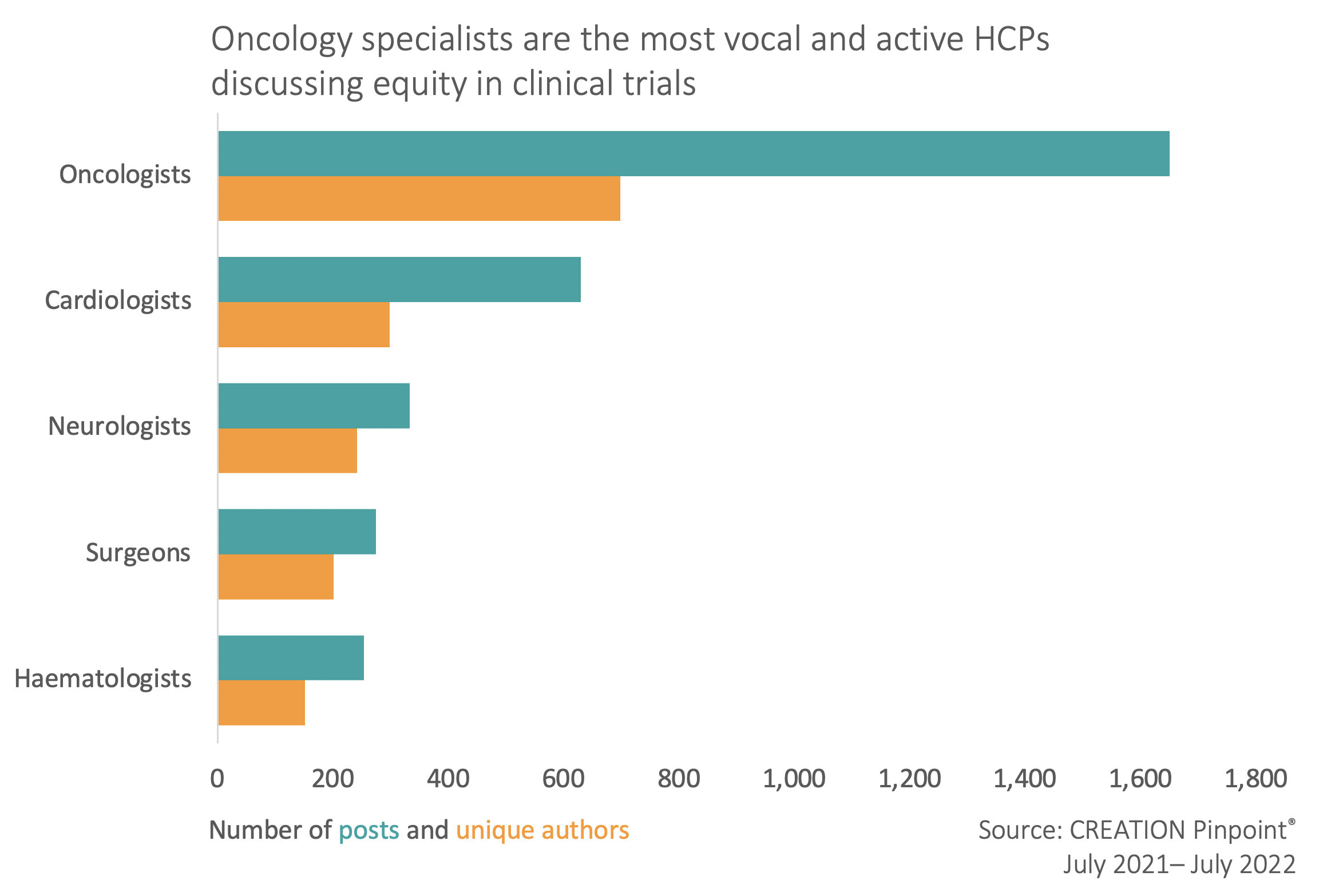
Segmenting this HCP conversation, it was clear that equity in clinical trials is discussed most prominently by those in oncology, suggesting this is a pertinent issue for a specialism with an abundance of active trials for new treatments.
Clinical trials are a fundamental way we make advances in cancer treatments. “Thank you” to every patient and their caregivers that have participated in a trial. You really have made a difference #ClinicalTrialsDay https://t.co/g5h5LEeZ2m
— Sanjay Popat (@DrSanjayPopat) May 20, 2022
Tackling under-recruitment
Clinical trials can only be proven successful when there are enough participants to provide statistically significant results, regardless of whether primary endpoints are met or not. HCPs have used social media to discuss concerns about under-recruitment, sharing potential solutions, including simplifying and demystifying trial design to help patients understand what they are signing up for.
Thanks for this abstract: We can’t fix #clinicaltrial enrollment #disparities until we:
▶️ simplify consent forms
▶️ make translated forms a requirement(I stilll don’t understand why it’s so expensive? Can we all agree on a common consent language?) #lcsm @n8pennell https://t.co/22cSsCRsTG
— Dr. Estela Rodriguez (@Latinamd) June 6, 2022
If you have a clinical trial going, consider @PRIMR_MED for patient recruitment and education.
So many trials close to under-recruitment, right? @stensy
Maybe this can help https://t.co/hZFhsv4pbL
— 𝙳𝚊𝚟𝚒𝚍 𝙲𝚊𝚗𝚎𝚜 (@CanesDavid) June 24, 2022
One approach taken by HCPs to directly tackle the issue of under-recruitment is to advertise their open trials online, such as psychiatrist Dr James Rucker, who posted a call for participants on Twitter. There were hundreds of similar posts from HCPs advertising clinical trials across a number of therapy areas.
We’re recruiting for our phase 1 clinical trial using 5 MeO DMT at the moment. We need healthy volunteers who haven’t taken a psychedelic before who’re available over the next few weeks for screening/dosing at King’s College Hospital, south London. If interested, please DM me 🙏
— James Rucker (@drjrucker) March 9, 2022
Some HCPs even advertised for trials they were not directly involved in but with which they had an invested interest. One such HCP was Georgia Ede, a psychiatrist exploring the connection between nutrition and mental health who posted about two trials looking at Alzheimer’s and the ketogenic diet. As HCPs build networks of like minded individuals online, they create a prime platform to find the ideal applicants for the trials they share.
https://twitter.com/GeorgiaEdeMD/status/1417641509235593217
Beyond sharing open trials seeking applicants, HCPs also disseminated articles to inspire peers to consider improvements to trial design to avoid under-recruitment issues. Hepatologist Nneka N. Ufere highlighted key points for cirrhosis trials including the use of shared-decision making tools, translated trial documentation and aiming to reduce administrative burden.
New piece by @silvio_silvio75 and colleagues in @APandT on how to improve recruitment and study design in IBD clinical trials – good food for thought when thinking about cirrhosis clinical trials. #livertwitter @ArpanPatelMD @hudsonbe81 @ebtapper @serpermhttps://t.co/jNIfJmqt04
— Nneka N. Ufere, MD MSCE (@NnekaUfereMD) February 8, 2022
Challenging disparities
Inequity in healthcare is an ongoing discussion among HCPs and other stakeholders as exemplified by ASCO’s 2021 annual meeting theme of ‘Equity: Every Patient. Every Day. Everywhere.’ CREATION.co has previously identified multiple areas of inequity HCPs are concerned with, including affordability, patient experience of care and clinical trial enrolment.
Important presentation by Dr. Debora Bruno @UHhospitals highlighting racial disparities in NGS testing and enrollment in clinical trials!! Need more efforts to ensure equal access to quality care!! #ASCO21 @ASCO @OncoAlert @montypal @PGrivasMDPhD@ASCOPost @ASCO_pubs pic.twitter.com/ytMAXSg3Xf
— Toni Choueiri, MD (@DrChoueiri) June 4, 2021
Some HCPs have used their online platforms to call to action those placed in a position to challenge existing paradigms, including Dr Ron DePinho, the past president of MD Anderson Cancer Center in Houston, Texas.
@theNASEM The need is 'urgent' to recruit more diverse participants for clinical trials. National Academies call for a paradigm shift that gives less power to institutions that fund and conduct clinical research and more to communities under study.
— Dr. Ron DePinho (@RonDePinho) May 18, 2022
Another behaviour observed is of HCPs calling out issues exposed by patient experience, such as Uché Blackstock, an emergency physician in New York who disseminated evidence that 40% of black women with metastatic breast cancer had not been offered clinical trial enrolment as an option in their treatment journey.
The majority of Black women with metastatic breast cancer don’t get enrolled into clinical trials. Only 40% of Black women said they were even offered a trial. One of the reasons Black women have a 41% higher death rate from breast cancer than white women.https://t.co/uAD7eX0av7
— uché blackstock, md (@uche_blackstock) June 6, 2022
Optimising trial design
As they seek to find solutions to challenge these existing paradigms in clinical trial recruitment, HCPs online provide suggestions to optimise trial design. One such HCP was Radiation Oncologist in training Rehema Thomas who, following an educational session addressing structural racism, asked a number of questions to consider when defining clinical trial design.
Important questions brought up during this thoughtful session. How are exclusion criteria contributing to inequity in clinical trials? Do certain ineligibility criteria overwhelmingly affect certain patient populations? Critical to think about during trial design. #DEIinRO #HEDI https://t.co/UYRQ3lf8ux
— Rehema J. Thomas, MD (@melaninandmed) November 3, 2021
At ASCO 2022, Haematology-Oncology fellow Adriana Kahn posted key points from a session regarding recruiting for trials in low and middle income countries.
Outstanding discussion on clinical trial design and solutions in #global #oncology! Key point was that we need to invest in local #cooperative group creation fostering national resources to make #LMIC truly active recruiters of international and locally-initiated trials! #ASCO22 pic.twitter.com/7PKe5gW2Fg
— Adriana Kahn, MD (@AdrianaKahnMD) June 6, 2022
As well as considering how trials are set-up, resourced and maintained to encourage recruitment, a number of HCPs drew attention to the repercussions of insufficient trial data. While some limitations are based on medical ethics and safety considerations, HCPs continue to discuss the implications on eventual patient treatment.
Artyom Korenevsky in Canada candidly explained that some paediatric patients are given drugs off label due to a lack of trials for their age group. He maintained that even though it is difficult to overcome enrolment risks, the threat of not having approved medication for these patients may be worse.
https://twitter.com/ArtKorenevsky/status/1429523613527465987
Another affected patient population is geriatric patients, identified in one instance by cardiologist Alfonso Valle, due to many trials only enrolling younger patients, meaning some new drugs don’t have ample evidence for use for those individuals.
⚠️New Drugs for #HF: What is the Evidence in Older Patients?
📌Trials enrolled younger 👥 vs clinical practice
📌registries confirming safety in older 👥 are needed
📌Older 👥 focused outcomes (functional status, QoL) are rarely assessed in clinical trials @secardiologia pic.twitter.com/o7TLTLzwhK— Alfonso Valle (@ValleAlfonso) August 6, 2021
Improving paradigms
Overall, Twitter presents a platform for HCPs to define and discuss areas for improvement for the collective goal of optimising clinical trials. The outcome benefits stakeholders beyond only HCPs, from the patient who will receive better treatments, and pharmaceutical companies who can complete more rigorous trials for drug development. By understanding concerns of the consumer in existing trial design, the pharmaceutical industry can work to revise their studies to comply with such requests.
These paradigms of limited recruitment criteria, issues with access to trial information and institutional biases are already being challenged by a number of HCPs and others seeking to challenge them.

 By Mary Kangley
By Mary Kangley