As many countries lift their lockdown restrictions, it might seem as though the conversation around COVID-19 treatment has lost momentum. After months of news about the pandemic, it would be natural to think that ‘COVID fatigue’ has begun to set in, and that online conversation is beginning to decline. We have seen that this is not the case for healthcare professionals (HCPs), who have continued to air their opinions, discuss with their peers and offer guidance to vulnerable patients.
Message on #COVID19 and #Dexamethasone
Note: For those with #Diabetes, read point 5 in particular
The main “weapons” continue to be social distancing and hand hygiene.
Thank you pic.twitter.com/8SJKPODmRX
— Partha S Kar 🇮🇳🇬🇧🏏🎥 (@parthaskar) June 16, 2020
Using the CREATION Pinpoint Engine, we are able to focus specifically on HCPs’ online conversations about coronavirus treatment, as opposed to talk about the pandemic generally, which is extremely prolific and diffuse. HCPs, like many of us, have a lot to say about how the pandemic has affected theirs and others’ lives, but in this article we will focus exclusively on conversation related to the medical treatment of COVID-19. We will look to discover how Pharma companies can maximise their impact and contribute constructively to the ongoing discussion that HCPs are cultivating.
HCPs discuss reactive, not preventative treatments
When looking at the conversations of HCPs on Twitter from 30 March to 1 July, there was a considerable difference in the volume of conversation about treatments as a whole and the proportion of that conversation that explicitly mentioned vaccines. Perhaps unsurprisingly, when HCPs talked about the reactive measures currently available to treat COVID-19, preventative measures that are still being developed (such as vaccines) were low down on the list of conversation topics. However, as we have found elsewhere, vaccines have been a hot topic outside the context of COVID-19 treatment. In total we discovered 21,000 posts from 11,000 HCPs related to COVID-19 treatments, but only 906 posts from 702 HCPs relating to vaccines within that treatment conversation.
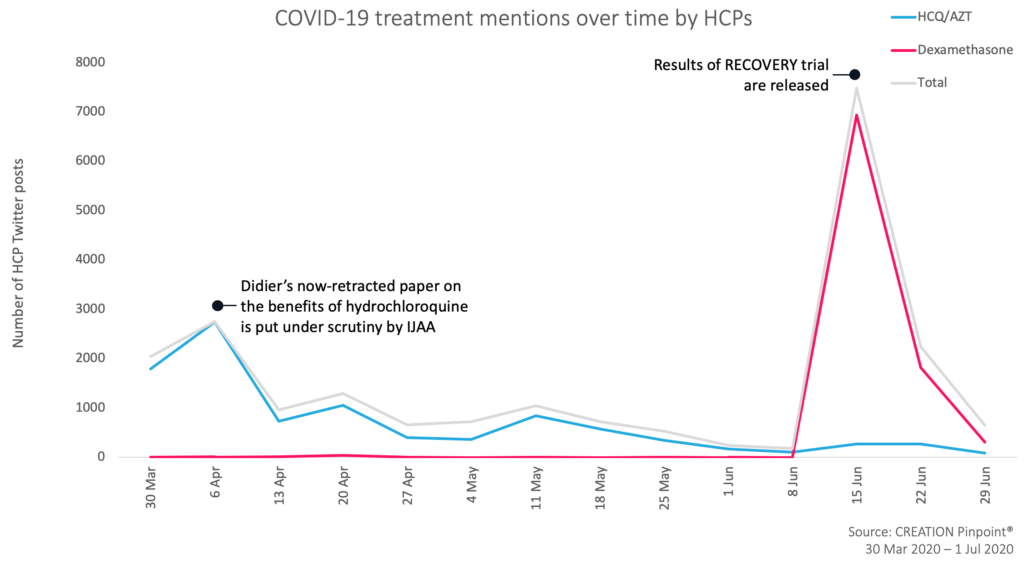
First and foremost, the HCP discussion of coronavirus treatments over the past few months is driven by real world data and trial results. All major spikes in the conversation since 30 March have been fuelled by ongoing developments, most notably following the results of the RECOVERY trial being made public. In early April, a similar spike was caused after Raoul Didier’s study “Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial” began to be placed under scrutiny from the journal that published it.
In this way, the HCP discussion of coronavirus treatment is grounded in and driven by real world events, particularly new trial data that presents evidence for or against a proposed coronavirus treatment.
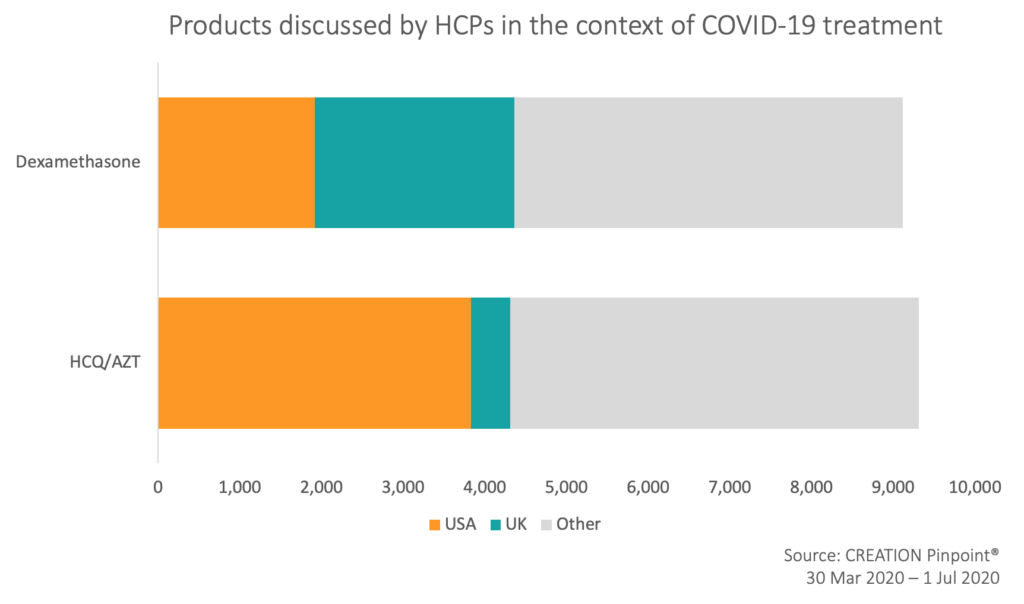
Another notable feature of HCPs’ COVID-19 treatment discussion is that whilst there are many candidate treatments for coronavirus, only a handful make up the vast majority of the online discourse .
Before news of the RECOVERY trial results, HCPs discussing COVID-19 treatment focused on a proposed combination of hydroxychloroquine and azithromycin. For the majority of the pandemic, it had been the most discussed treatment for COVID-19 by a wide margin. HCP discussion about this drug combination has been marked by controversy following an endorsement by Donald Trump, with some HCPs defending its use, whilst others urge caution due to evidence that it lacks efficacy and increases the risk profile of some patient groups.
WHAT HCPs THINK IS IMPORTANT
When reacting to and assessing new trial data for potential COVID-19 treatments, HCPs also focused on practical areas of concern, such as the cost of administering treatment and accessibility for patients. In particular, dexamethasone’s low cost and ready availability made it the target of favourable comparisons with Gilead’s remdesivir:
The Gilead bill arrives ⬇️ despite assurances there would be no profiteering & the (weak) evidence coming from an NIH funded trial. >$2000 for 5 day course! Thankfully irrelevant since 10 days of Dexamethasome costs £5… & actually reduces your risk of dying (by a lot) #COVID19 https://t.co/SW1GmhhFU6
— Kevin Blyth 💙 (@kevingblyth) June 29, 2020
Many HCPs demonstrated an awareness and concern for the challenges faced by patients.
One reason why impact of dexamethasone on death in sick covid cases in ‘recovery’ trial is big news for india? Becos its available ‘today’ in hosp across India at low cost
If used in right patients with eye on sideeffects it can save lives today irrespective of ability to pay— sanjay nagral (@sanjaynagral) June 17, 2020
In this way, COVID-19 treatments that can be easily administered are viewed favourably by HCPs, as they minimise the barrier to access for patients.
This concern may be a reason why treatments that ameliorate the symptoms of a severe reaction to coronavirus (such as dexamethasone) are talked about so widely in comparison to preventative measures, such as one of the many vaccine trials. Before a vaccine is widely available, it is understandable that HCP’s concern may lie with those who have already caught the disease, and are experiencing severe symptoms.
Moreover, HCP discussion of COVID-19 treatment is dominated by products that are already being used in hospitals, and since a working vaccine is not currently available, many HCPs have expressed a wish to focus on reducing deaths with the resources that are available to them.
In this way, HCPs express an awareness of limited hospital resources for those who develop severe COVID-19 symptoms, and acknowledge that expensive treatments could leave the most vulnerable excluded.
Another notable feature of the online HCP discussion around treatments for COVID-19, is that US and UK HCPs make up about half of the total posts in the discussion, though when looking at individual products, there is some regional variance. Whilst HCPs in the US made up most of the HCQ/AZT discussion, UK HCPs were the largest share of voice for dexamethasone. Interestingly, HCPs in the same country as a study were the most likely to talk about it online, as with UK HCPs and the RECOVERY trial results, which took place in the UK. HCPs in France were the next most prominent share of voice, made up largely of reactions and discussion surrounding Raoul Didier’s study of hydroxychloroquine, which took place in France. In this way the dataset appears to suggest that HCPs are more likely to talk about studies that are local to them.
How can Pharma support HCPs in the COVID-19 treatment conversation?
Direct engagement with Pharma companies by HCPs was rare, despite extensive discussion of Pharma products. Some corporate content was shared however. HCPs were particularly receptive to educational content, such as webinars.
Nuestra Directora General, Constanza Losada, estará el 16 de junio en el webinar de @TheTOPCompanies hablando sobre el valor de la innovación farmacéutica frente al COVID-19. La cita es a las 5:00 pm en la siguiente liga: https://t.co/OHqFWvOBXP pic.twitter.com/pHIuX0pQ4J
— Pfizer México (@PfizerMx) June 12, 2020
In this way, HCPs chose to engage with corporate content that offered up something helpful or relevant to their immediate needs.
By identifying what HCPs are interested in and concerned with in the COVID-19 crisis, Pharma can earn their support and attention. Since HCPs in many of their discussions are focused on the needs of patients, identifying how HCPs can meet these needs could be a through-road towards more engagement between HCPs and Pharma.
For example, the data has demonstrated that online discussion is driven by events and milestones related to the treatment of COVID-19, as has been seen with news of the RECOVERY trial. As a result, Pharma is in a unique position to support HCPs through their corporate communications, provided they have a good understanding of HCPs’ online behaviours.
Conclusion
In summary, the last few months of COVID-19 treatment discussion by HCPs on Twitter has been driven by new trial data from studies exploring the efficacy of potential COVID-19 treatments. Overwhelmingly, the candidates receiving the most attention are low cost and readily available hospital products that aim to ameliorate the symptoms of severe COVID-19 cases.
Additionally, US and UK HCPs make up about half of the treatment discussion globally, with UK HCPs in particular taking up a disproportionately large share of voice. Moreover whilst HCPs regularly share news of vaccine progress, they also focus on challenges that face them presently, and are more likely to engage directly with educational content than any other kind of content currently posted by Pharma on social platforms such as Twitter.
With a developed understanding of HCPs’ online behaviours, and an awareness of how the online conversation is changing over time, there is a significant opportunity for effective communication between HCPs and Pharma, at a time when information about COVID-19 treatment and how we can best respond to the crisis is in high demand.
For more insights into the conversations that HCPs are having in relation to COVID-19 you can read more of our public content or get in touch, we’d love to help.
Methodology:
- the data used was taken from global Twitter posts of HCPs, in multiple languages, from 30th March to 29th June 2020.
- The dataset’s scope was limited to mentions of products or language relating to the treatment of COVID-19. As such, the information in this article only reflects a subset of the whole online COVID-19 conversation, albeit one of particular prominence for HCPs working on the frontline of the pandemic today.