31.01.2023 | Insight
Lecanemab: Full phase 3 trial results for the Alzheimer’s disease drug not as positive as HCPs expected
Earlier this year, CREATION.co published an article on the approval of Aduhelm (aducanumab), a monoclonal antibody that targets amyloid beta found in the brains of people with Alzheimer’s disease, and which was also the first novel therapy approved for the disease since 2003.
With the landscape of treatment now changing, particularly with the FDA accelerated approval of Eisais’ new Alzheimer’s disease drug, Leqembi (lecanemab-irmb), CREATION.co was prompted to follow up on what HCPs are saying in the online conversation about Alzheimer’s disease. CREATION.co analysed over 45K English language posts by nearly 12K distinct HCPs online from March 14th 2022 (following on from the end of the research period from our previous article) to December 11th 2022.
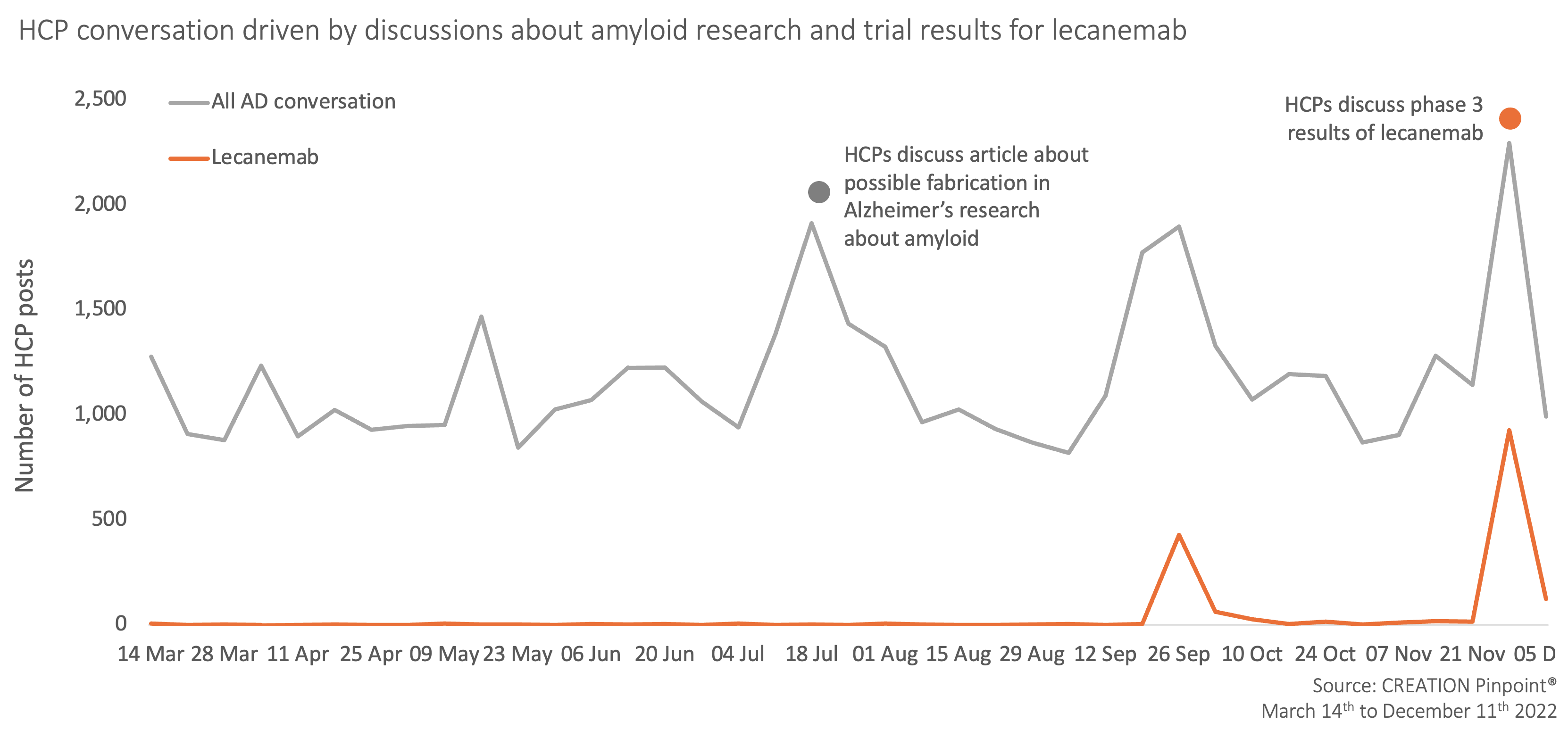
Since CREATION.co’s previous Alzheimer’s disease article, the volume of online HCP conversation within the therapy area has remained consistent. Peaks in discussion surrounded an article that discussed the discovery of possible fabrication of images that could disprove the amyloid hypothesis, threatening the scientific progress in curing or slowing down Alzheimer’s disease.
Phase 3 trial data for lecanemab has been a key driver of HCP conversations, with HCP sentiment shifting from positively celebrating initial trial data at the end of September 2022, to negative sentiments more recently.
End of September: HCPs and news outlets celebrate Biogen and Eisai’s announcement of phase 3 trial results of lecanemab
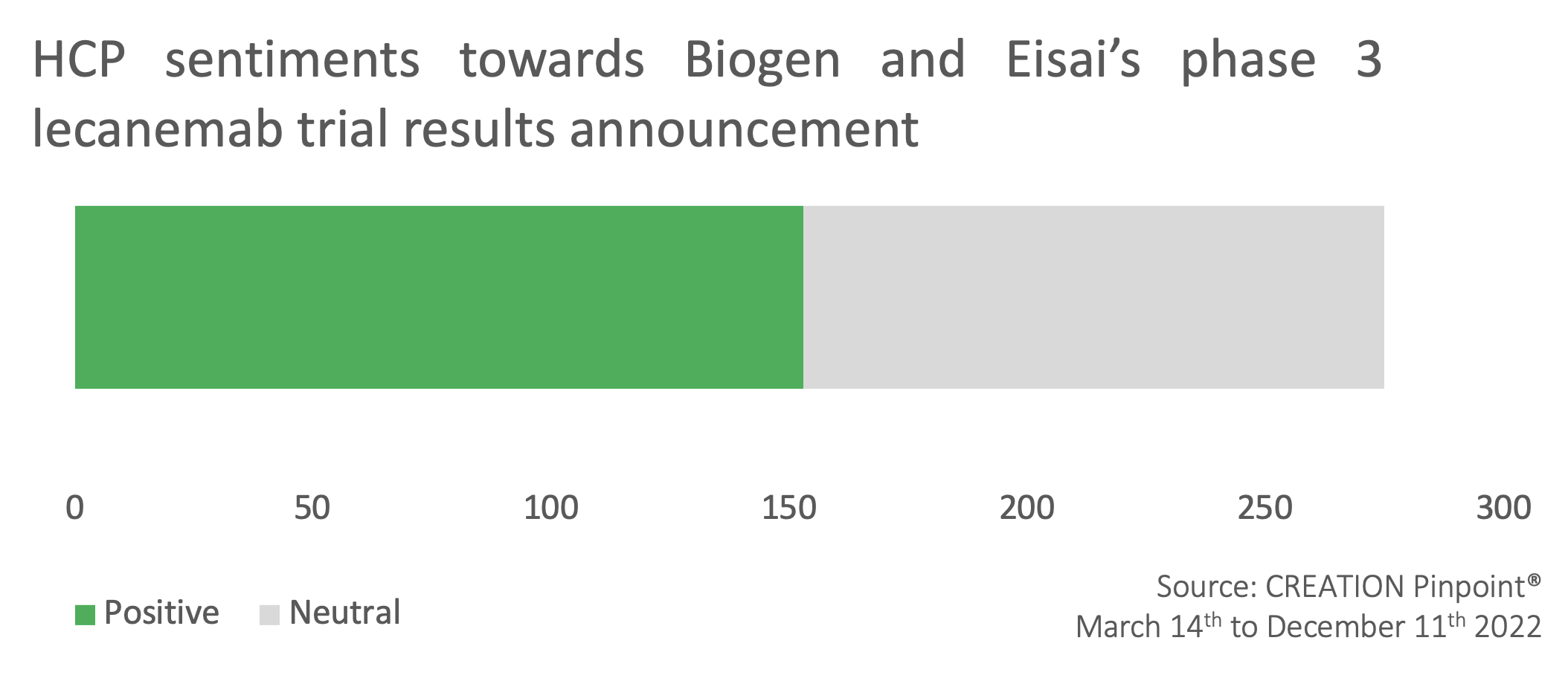
Over half of HCP posts concerning the announcement of the phase 3 trial results of lecanemab were positive, with HCPs celebrating it as a ‘milestone’ and ‘huge news’. HCPs particularly celebrated that the drug met its primary endpoints.
Huge Alzheimer’s news; new drug! Phase 3 confirmatory Clarity AD clinical trial of lecanemab, investigational anti-amyloid beta (Aβ) protofibril antibody, met primary endpoint (CDR-SB: Clinical Dementia Rating-Sum of Boxes) and key secondary endpoints. Great news! @FixelInstitute pic.twitter.com/vYSKJy9MX3
— Michael Okun (@MichaelOkun) September 28, 2022
Important milestone for AD clinical research today with Phase 3 lecanemab trial result, which sounds likely to meet FDA approval threshold for early AD. How did we get here and what could be next? …1/n https://t.co/WotAdd1uAL
— Kathy Liu (@kathy_y_liu) September 28, 2022
When HCPs expressed a less positive sentiment, they primarily cautioned against excitement before a release of the full data and discussed the possible issue of toxicity.
💊🧠Benefit of #lecanemab in #Alzheimers below "clinically meaningful", aka very small or meaningless? @AlbertoEspay is spot on.
⚡️Also, toxicity still a big issue:
➡️ARIA incidence 21% (@microbleeds /edema)We need to wait for the full data!!#neurotwitter #dementia @EricTopol https://t.co/mYJjovUJjt pic.twitter.com/KMjHsdhk5d
— Andreas Charidimou MD, PhD (@a_charidimou) September 28, 2022
Initial report from trial of lecanemab: statistically met endpoints, but clinically the effect is very small and toxicity is still an issue. Be careful of the hype before we see the data in full. There is a long history here. #endalz https://t.co/MH5UMqZbm8
— Matthew Schrag (@schrag_matthew) September 28, 2022
Lecanemab results show some potential, but not enough info yet to work out whether it's useful – effect size is too small to be convincing without seeing more data (why don't people do Bayesian stats!). Reassuringly it is being trialled with a tau therapy – I'd like to see that.
— Timothy Rittman (@timrittman) September 28, 2022
Following release of the full trial data: HCP sentiments shift from largely positive towards increasingly negative
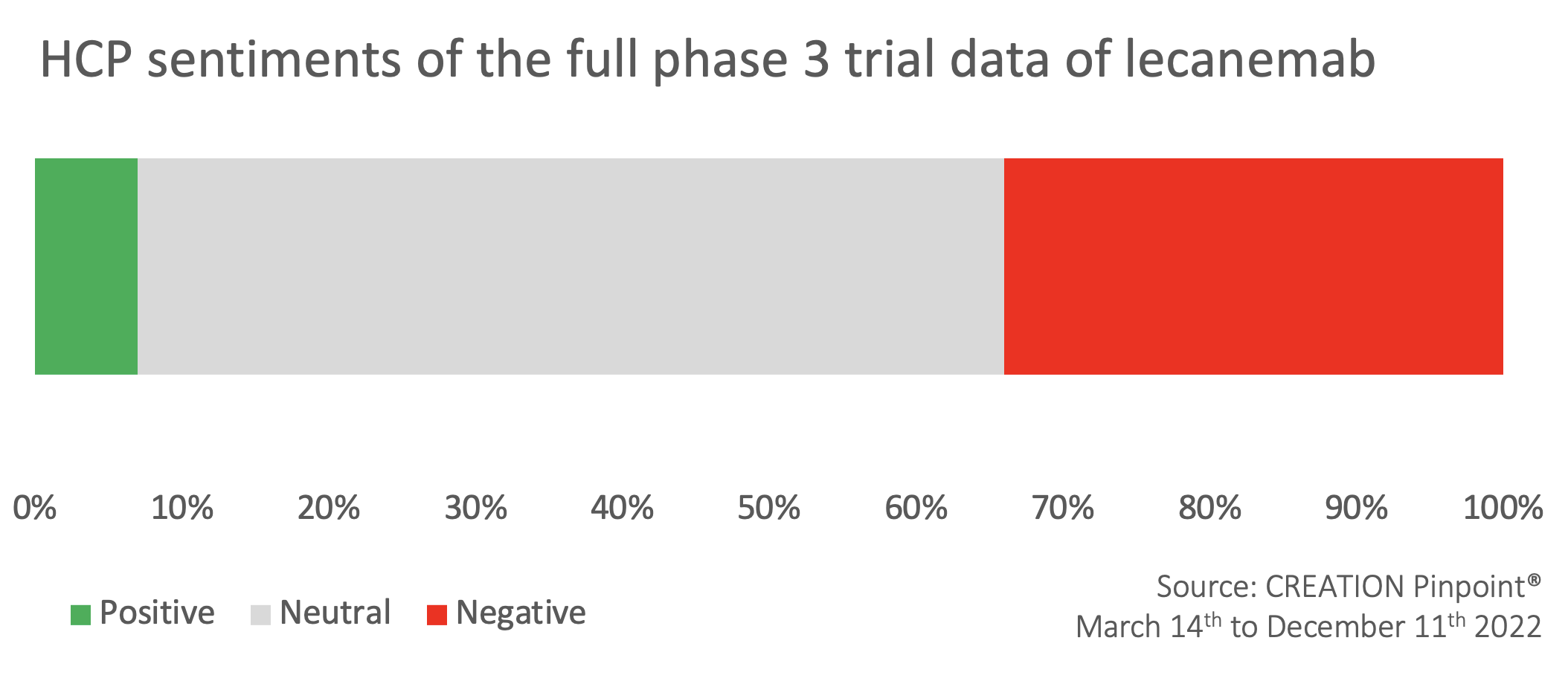
Compared to the largely positive sentiment following the initial announcement, HCP sentiment towards lecanemab became more negative following the release of the full trial data, with under 10% of sentiment remaining positive. US neurologist Matthew Schrag called the data ‘not a breakthrough’, explaining in a thread that the actual benefits were ‘tiny’ and ‘will probably not be detectable’.
My thoughts on the phase 3 results of Alzheimer's drug lecanemab from Biogen and Eisai, presented at #CTAD #CTAD22 and published this evening in the @NEJM. Major takeaway – this is NOT the breakthrough we have been waiting for. Key points: pic.twitter.com/zPDSx5BEcL
— Matthew Schrag (@schrag_matthew) November 30, 2022
Expressing a similar sentiment, Eric Topol shared a blog on science.org, highlighting the statement that expressed doubt that lecanemab “will actually make a difference in the treatment of Alzheimer’s”.
Best analysis of the trial data, and contextualizedhttps://t.co/ozw7uSlBIb
by @Dereklowe pic.twitter.com/w6Rfp39TKO— Eric Topol (@EricTopol) December 1, 2022
Negative sentiments was also focused around the high risk of adverse effects observed during the trial, with Eric Topol highlighting his concern at the death of a patient on lecanemab, and neurologist Andreas Charidimou listing associated adverse events compared to the negligible clinical benefits:
Very concerning about the new drug w/modest effects for Alzheimer's disease (AD)
"If the patient hadn’t been on lecanemab she would be alive today.”
Underlying cerebral amyloid angiopathy is present in nearly half of AD patientshttps://t.co/UNRoKcICNw @cpiller @ScienceMagazine— Eric Topol (@EricTopol) November 28, 2022
Agree w @schrag_matthew:
✍️Absol. clinical benefit negligible (Δ2.5%)
⚠️21% brain swelling/bleeding #ARIA
🩸2 fatal🧠bleeds w blood thinners/tPA
🚀Anticoagulation &🧠bleeds⬆️real world?
🛑Amyloid angiopathy #CAA pts on OAC shouldn't take #neurotwitter https://t.co/yIV0ea6USc pic.twitter.com/TEE3I5dXlF— Andreas Charidimou MD, PhD (@a_charidimou) November 30, 2022
Important to listen to HCPs, and not be drawn into hyperbolic reporting
Amidst the clinical conversation, British psychiatrist Professor Rob Howard highlighted the effect on patients of the hyperbolic reporting of the lecanemab trial data, a perspective often overlooked by clinicians and the media. Professor Howard shared the desperation of his own patients and his own experience of receiving calls in his clinic. He also shared his own official comment on the trial results in which he stated that he “…[suspects] that the lack of demonstrable clinical effectiveness will mean that lecanemab will not be taken up widely within healthcare systems around the World…” Importantly, he also shared a blog post by the adult child of a patient which stated, “…for anyone living with the condition now, it offers no immediate hope.
What I resent is the way this fact gets buried in the hyperbole, the false hope it generates, and the way it displaces efforts to do other – non-pharmaceutical – things that could be equally if not more immediately beneficial at improving people’s experience of living with dementia.”
Does he take Lecanemab? Blogged: https://t.co/wf1mIoEY2a
— Neil Crowther (@neilmcrowther) December 1, 2022
Keeping the pulse on HCP conversation in Alzheimer’s disease
Despite lecanemab receiving accelerated FDA approval, HCP discussion online on the phase 3 trial results suggest that many HCPs believe that there is a long way to go before significant progress is made. HCPs celebration of the announcement of the initial trial results shifted towards concern and doubt as the full results were published, with clinical significance and benefits and adverse events leading HCPs to feel less than positive about lecanemab. As new developments occur in this therapy area, CREATION.co will continue to monitor HCP conversation to find out what they are saying.

 By Francesca Gan
By Francesca Gan