With Eli Lilly’s Emgality and Teva’s calcitonin gene related peptide (CGRP) inhibitor approved hot on the heels of Amgen and Novartis’ Aimovig, and with other CGRP offerings nearing approval, the preventive treatment of migraines is becoming a key topic. We analysed the online conversation of healthcare professionals (HCPs) in the United States to understand their perspective on the progress of the new generation CGRPs1. HCP excitement about positive product pipelines simmered throughout the first half of 2018, but the discussion really took off in May 2018 around the time that Novartis and Amgen’s joint venture, Aimovig, received FDA approval.
HCPs prepare for breakthrough
Shortly before FDA approval, doctors demonstrated a desire for information and guidance regarding the use of the new products. A paper published on May 7 by the Journal of the American Medical Association entitled ‘Who Should Try New Antibody Treatments for Migraine?’, was shared 80 times, including 29 shares by US HCPs as they looked to orientate themselves around the promising treatment potential.
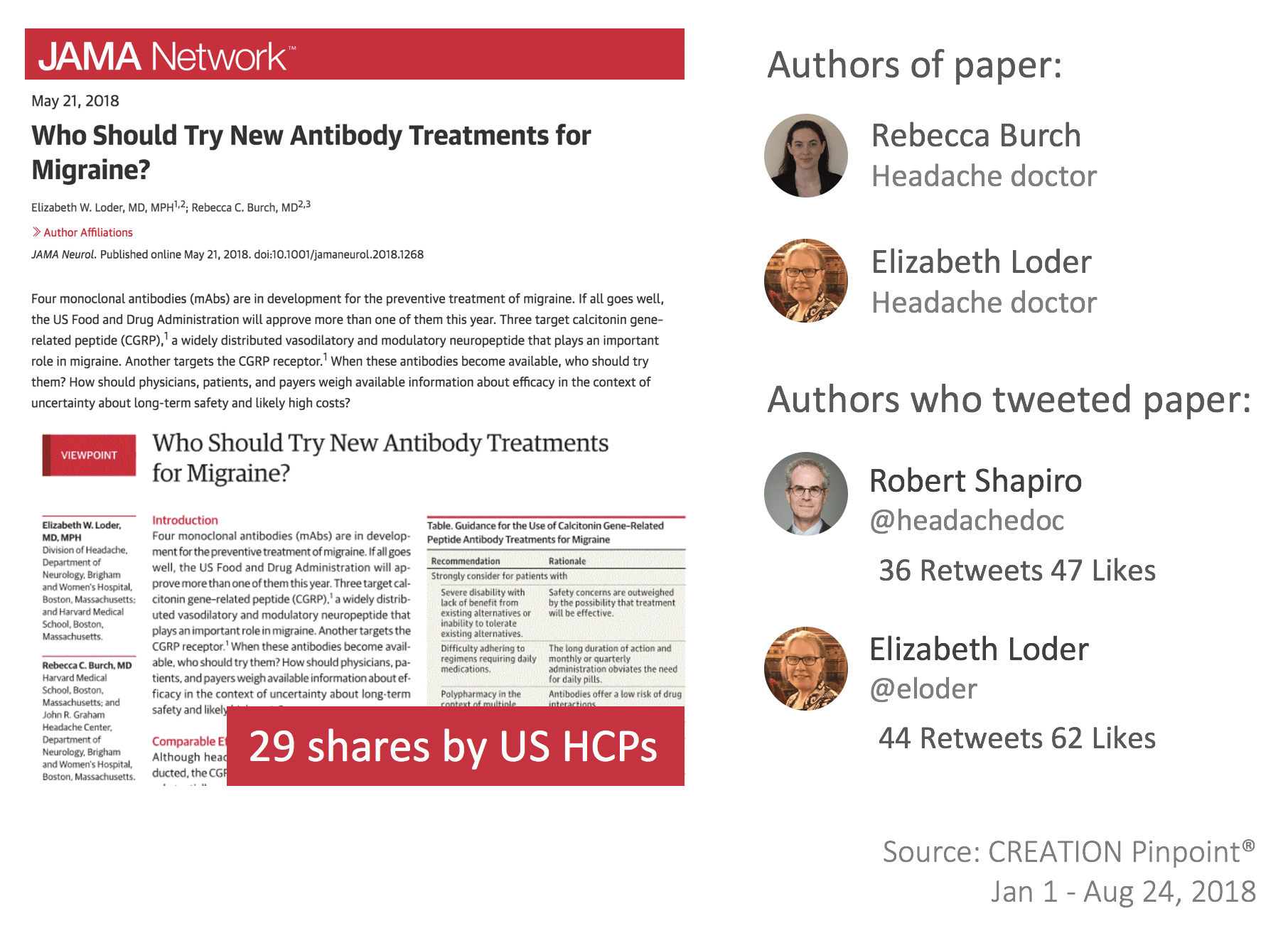
The paper presented guidance regarding the profile of migraine patients suitable to receive the class and addressed HCP concerns around unknown long-term safety and cost-benefit ratio. However, despite the general feeling of positivity, there was evidence that some doctors felt concern about CGRPs.
A good drug that no-one can access?
Despite promising data around CGRP inhibitors, it soon became clear that all was not well in the minds of HCPs. Dallas-based neurologist, Bert Vargas was one of the first to open the issue of access as he responded Aimovig approval. Vargas’ thoughts seemed to resonate with the general consensus as peers and other interested parties engaged with the post.
#Aimovig is finally FDA approved for #migraine. The question now will be whether it will be accessible to those who need it. https://t.co/wCtswXDJwL
— Bert Vargas (@BertVargas) May 19, 2018
Whilst some stated similar concerns regarding Aimovig, others extended the complaint to encompass the entire class.
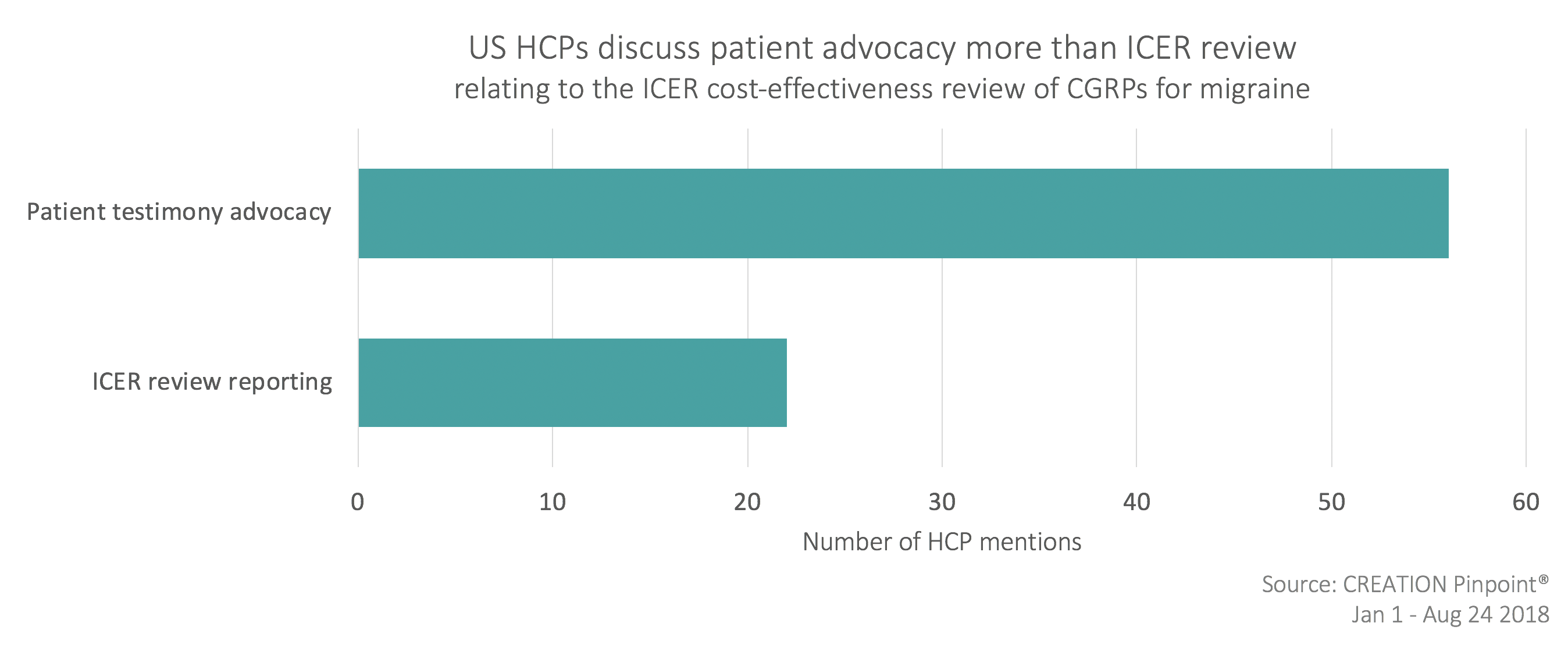
A feature of US HCPs’ conversation was ICER’s draft report – a review of the cost-effectiveness of Aimovig. Prior to the 14th June report, HCPs and advocacy groups called upon others to share their stories for consideration in the upcoming review by using hashtags #Migraine #advocates and #MigraineWarriors.
Despite the lower than expected price of Aimovig, there was still concern that this would not meet ICER’s expectations compared to other treatment options.
#Migraine #advocates need to mobilize! Last chance to share your story of living with #migrainedisease to impact access to #CGRP medicines. Deadline for input is TUESDAY, MAY 8. #migrainelife #chronicmigraine #trustpatients https://t.co/szar1PVLuM pic.twitter.com/cIwbRElyIR
— Jaime M Sanders | The Migraine Diva (she/her/hers) (@migrainediva) May 7, 2018
HCPs were cautiously optimistic in the build-up to the ICER recommendations and met the report with positivity, however there was a sense of disappointment when the drug was suggested primarily for patients already unsuccessfully treated with other migraine options. Despite ICER’s cost-effective recommendation, access concerns persisted, with high-cost the primary worry. HCPs continued to support the patient position by sharing posts commenting on the ‘powerful’ patient testimonies and encouraging insurance companies to reign in access controls.
Insurance companies were not the only party to take the blame of the high cost of the new migraine treatments. HCPs also indicated pharmaceutical companies’ role in the cost of medications.
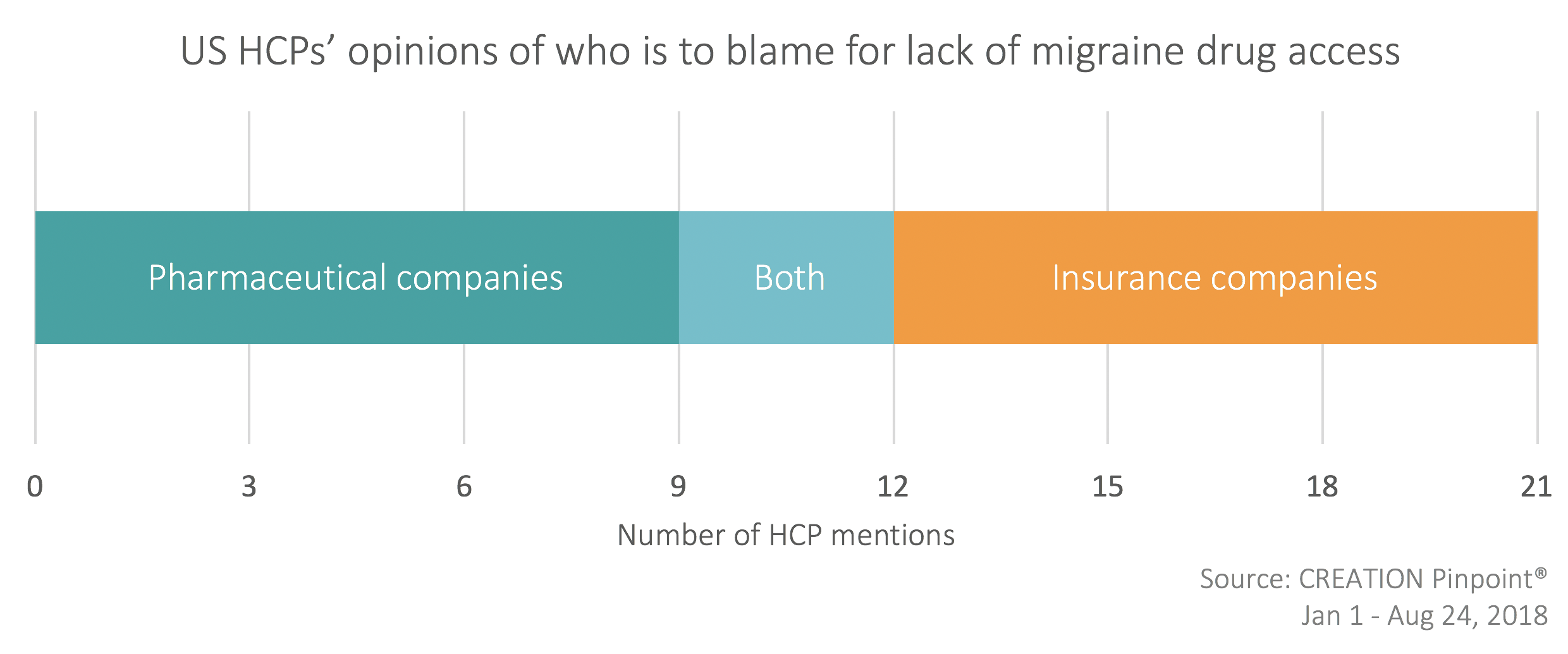
Migraine specialist Amaal Starling was not alone in suggesting that insurance companies’ decision to restrict prescribing to specialists was the primary reason for reduced CGRP inhibitor access.
In response to this, Matthew Robbins, a neurologist in New York, agreed the significance of insurance company restrictions, but added that high drug pricing enforced by the pharmaceutical industry is of far greater concern.
More problems at the S. Korean manufacturing plant for fremanezumab (Ajovy) anti-CGRP mAb for #migraine and cluster #headache.
FDA's decision is reportedly still on track for Sept 16th, but when will the drug actually be available if it's approved?https://t.co/lFA4HZZYSX— Robert Shapiro (@headachedoc) August 17, 2018
This is a very important issue about restricting access to #CGRP mAb therapies for #migraine and glad @NeurologyToday @BrainHealthMD are investigating.
However, one issue so frequently omitted: what drives the lack of access? The high cost of medication set by industry. https://t.co/fF5V1uEObw
— Matthew Robbins, MD (@mrobbinsmd) July 7, 2018
Breakthrough for women’s health or slow and sexist?
Despite these concerns, CGRPs are being heralded as a breakthrough specifically for women’s health. However some HCPs questioned why such a significant advancement took so long to materialise. As women are the primary sufferers of migraines there is some suggestion that sexism has come into play. Aimovig manufacturer Novartis prepared a response to these claims. Seven HCPs shared Novartis’ plans to use messaging in the marketing of Aimovig to counteract the concerns relating to sexism which could result in lack of drug adoption.
However, headache doctor Elizabeth Loder, amongst others, was critical of this approach, suggesting that Novartis was taking advantage of feminism and needs to re-evaluate its pricing rather blaming sexism for lack of access.
This is Big Pharma exploiting feminism. It is not sexist to impose rational access controls on high priced new #migraine medicines. Reasonable pricing is the answer to this problem. That ball is in your court, @Novartis https://t.co/yodufanSOa
— Elizabeth Loder, MD, MPH (@eloder) June 30, 2018
How do CGRP inhibitors compare with current treatments?
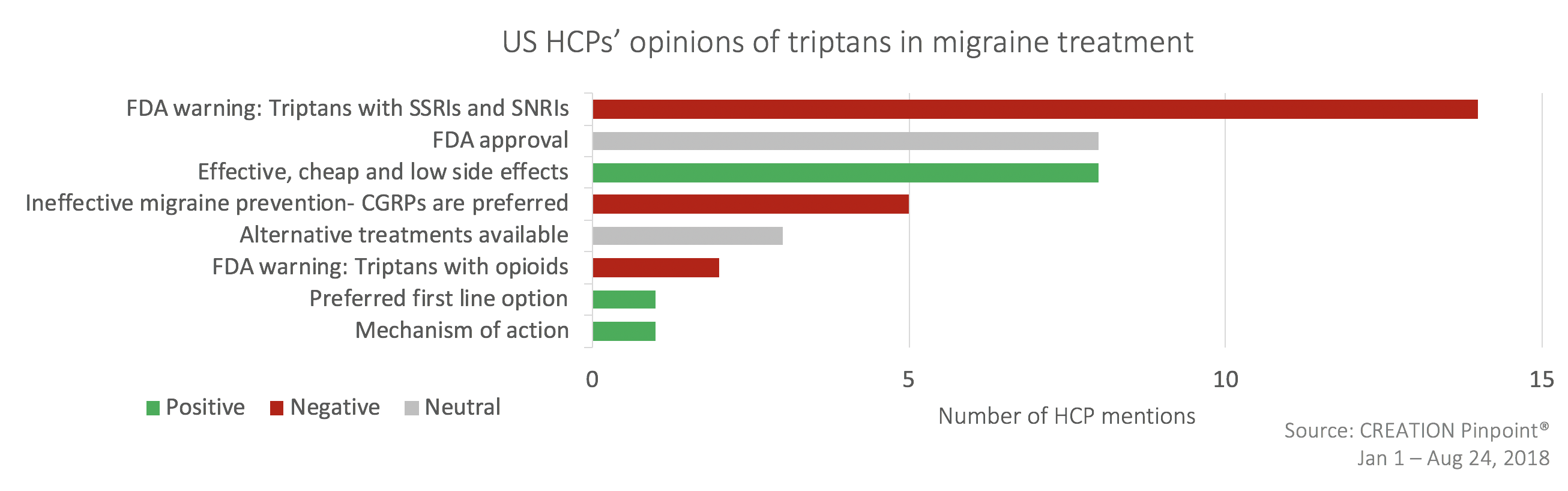
Current first line treatments may have a difficult task at hand to maintain market share as HCPs found fault in the triptans; FDA warnings regarding the coincident use of triptan alongside SSRIs, SNRIs and opioids were shared and some suggested that CGRP inhibitors may be a suitable replacement.
Dr. Lipton: >1/3 of those with with migraine do not respond to triptans or have contraindications. Rimegepant 75 mg, a CGRP-antagonist for acute migraine, proved effective vs placebo for acute migraine. A potential well-tolerated alternative to triptans and NSAIDs. #AHS18SF
— Jeff Headache Ctr (@JeffHeadacheCtr) June 30, 2018
However, the jury is still out for the new class with reports that they may not be appropriate for all.
This is one misinformed analysis (e.g. conflates NSAIDS with beta-blockers, triptans with CGRP mAbs, etc).
Though… true enough that only some will likely gain much benefit from Aimovig.
Hopefully those not benefiting will stop it when that becomes clear.https://t.co/C2htttnNh2— Robert Shapiro (@headachedoc) June 7, 2018
Positive treatment potential confounded by pricing and marketing issues has left HCPs considering how the new class compares to current migraine options, and how it may fit into the treatment paradigm. Despite the skepticism reported here, CGRPs look to have won over many HCPs, and could well become a key feature of the migraine treatment landscape over the coming years.
To find out more about how online HCP insights can inform brand strategy and shape positioning sign up for an educational webinar at creation.co
Notes:
- Data to inform the analysis in this article was researched using CREATION Pinpoint, the HCP insights platform from CREATION. Analysis showed that 760 HCPs in the United States were actively discussing migraine treatment online, posting almost 2,000 times in the first 8 months of 2018.
 By Jamie Doggett
By Jamie Doggett 



