Discover what healthcare professionals (HCPs) think about pharmaceutical products and their manufacturers, as it happens, through CREATION.co’s tracking updates. How are HCPs responding to the latest trial results for COVID-19 vaccine candidates? How are HCPs talking about or engaging with the Top 50 pharma companies on social media? Each week CREATION.co’s tracking updates bring you the latest insights from the conversation of HCPs across the globe discussing these topics and more.
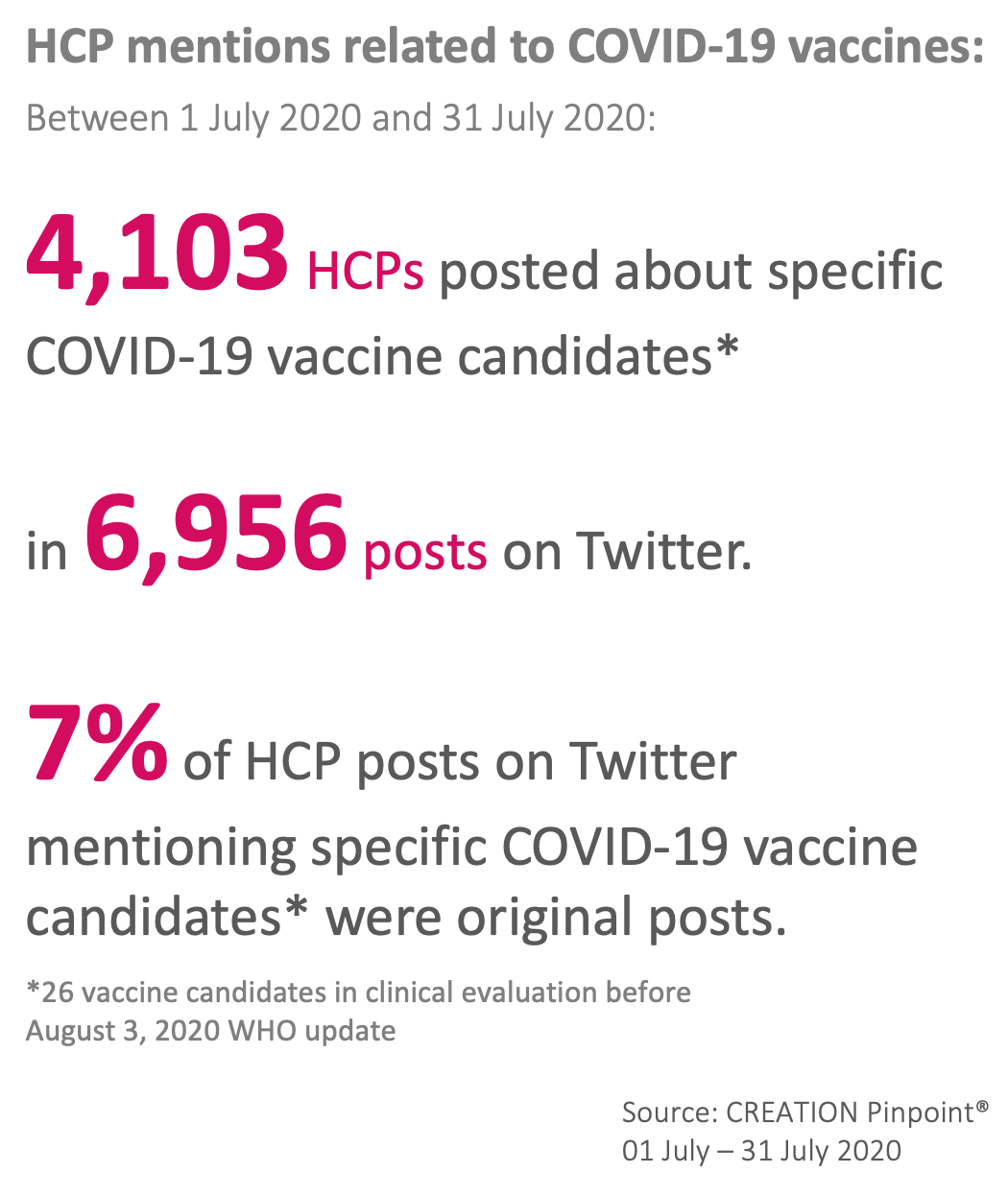
The Top 10 covid-19 vaccine candidates mentioned by HCPs on Twitter in July
Out of 26 candidates in clinical evaluation during July, HCP discussion focused mostly on collaborations between University of Oxford and AstraZeneca which was the most mentioned vaccine candidate, as well as Moderna and NIAID. Compared with the previous tracking period, the top 3 most discussed COVID-19 vaccines remained the same, while new players entered the top 10.
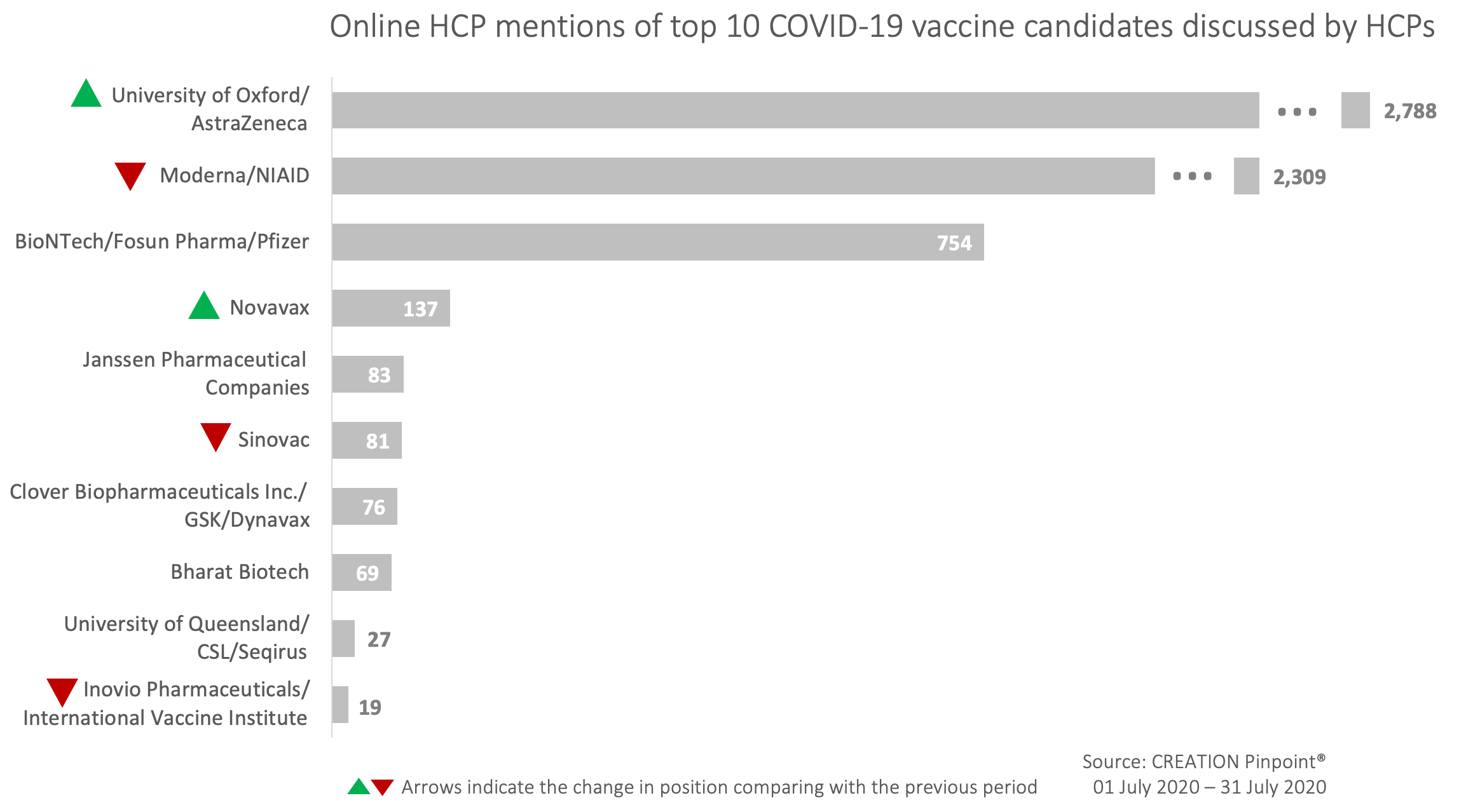
July’s insights from HCPs mentioning covid-19 vaccine candidates on Twitter
During the month of July, the HCP vaccine candidate conversation level on Twitter has decreased slightly when compared to June, yet was still driven by the advancements in study data and new trial stage announcements.
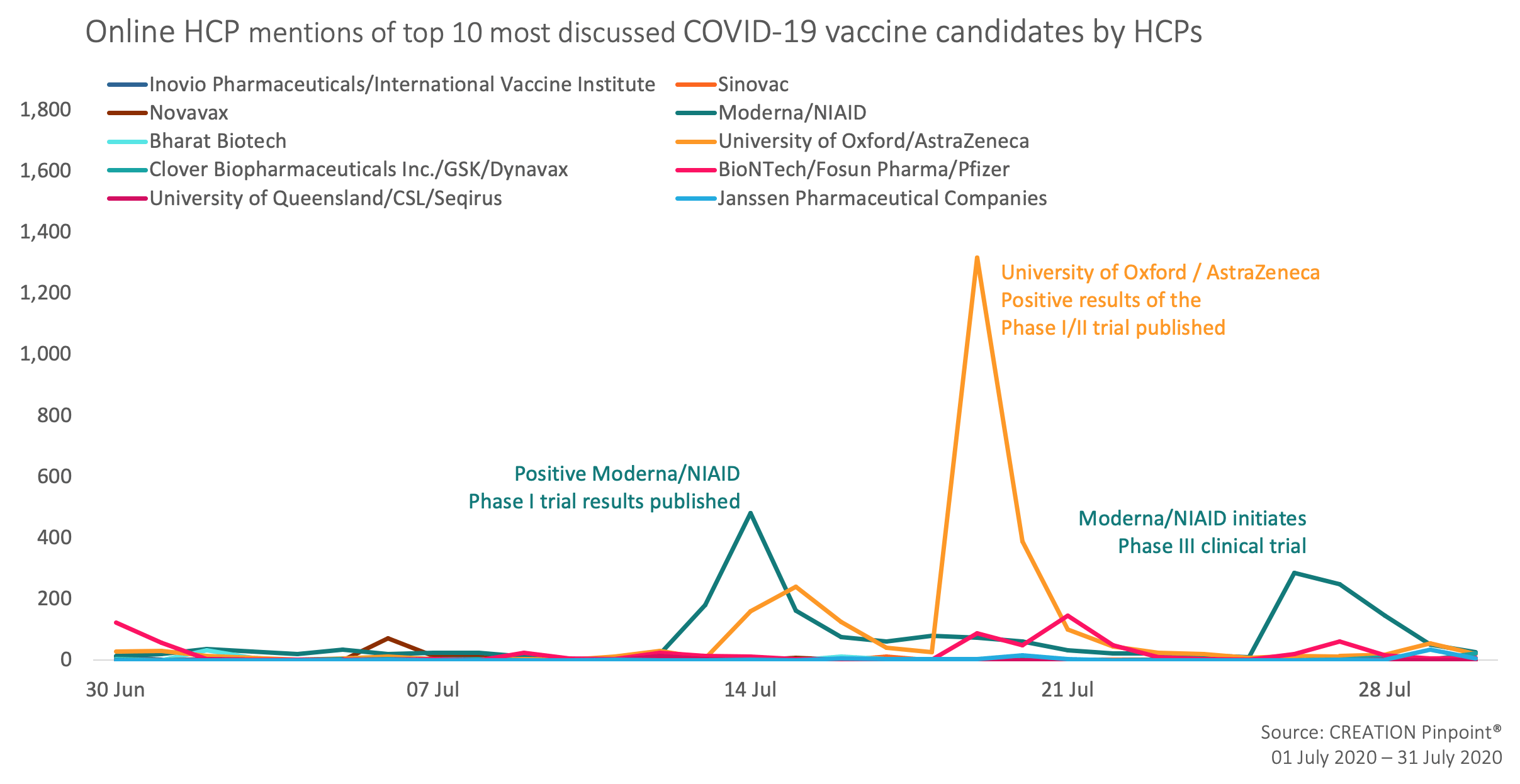
The University of Oxford and AstraZeneca’s Phase II success stirred up the most HCP conversation around the world in July. HCPs mentioned the vaccine in nearly 3,000 posts, mostly sharing the good news of the vaccine being safe and well-tolerated from the University of Oxford and BBC Twitter accounts. The post by Richard Horton, the chief editor of the Lancet medical journal, was the most engaged with by HCPs, resulting in over 200 retweets.
The phase 1/2 Oxford COVID-19 vaccine trial is now published. The vaccine is safe, well-tolerated, and immunogenic. Congratulations to Pedro Folegatti and colleagues. These results are extremely encouraging. https://t.co/oQp2eoZYIg
— richard horton (@richardhorton1) July 20, 2020
Results of a Phase I/II clinical trial for the Oxford Vaccine:
>1000 people randomized – #COVID19 vaccine vs
meningitis vaccine (placebo)-Antibody responses look good
-Booster dose improved antibody response
-No serious adverse eventsExciting times!https://t.co/zcAuy1HPR1
— Isaac Bogoch (@BogochIsaac) July 20, 2020
HCPs also shared “very encouraging” University of Oxford/AstraZeneca trial data published in the Lancet Medical journal that was shared by an American cardiologist Eric Topol. A Bloomberg article which stated that the vaccine is “months ahead” of its competitors and told a story of Sarah Gilbert, the lead researcher, had caught HCPs’ attention too.
It’s now becoming clear that the Oxford led vaccine is the front runner in the world vaccine race. Already in phase 3 trials in Brazil. It was initially even tested by researcher Sarah Gilbert’s 3 children – 21 year old triplets who volunteered. Good read. https://t.co/CHHGNB0AWf
— Eric Feigl-Ding (@DrEricDing) July 16, 2020
Moderna/NIAID positive Phase I trial results published in NEJM were labeled by HCPs as “promising” and the whole development – “amazingly fast science”. The second spike in the HCP Moderna/NIAID vaccine mentions was caused by sharing the news of the vaccine advancing into Phase III in the end of July.
Moderna begins 30,000 participant Phase 3 COVID19 vaccine trial TODAY. The trial will evaluate safety in various demographics & will evaluate ability to prevent or lessen severity of infection. This may provide the info we have been waiting for regarding antibodies and immunity.
— Nicole Saphier, MD (@NBSaphierMD) July 27, 2020
Overall, HCPs showed positive attitude towards the vaccine candidate development, with only a minority showing concerns for the cost and discussing the manufacturer profits.
We will be tracking the online HCP conversation to identify trends and change in views relating to COVID-19 vaccines. You can stay up to date with HCP insights by subscribing to CREATION Knowledge e-journal.
I was glad to see @pfizer put up their phase 1 trial data today. Virus neutralizing antibody titers achieved after two doses are greater than convalescent antibody titers. Maybe the first objective good news we've seen out of Operation Warp Speed?https://t.co/uzp4bKkpAm
— Prof Peter Hotez MD PhD (@PeterHotez) July 1, 2020
Very good news from the Oxford vaccine trial. Glad to see both antibody and T cell responses.
How glad? Well sign me up for the vaccine!I’m hopeful for a vaccine before end of 2020 atleast for vulnerable populations. Kudos to @UniofOxford @AstraZeneca https://t.co/BRVuA9Cg88
— Vincent Rajkumar (@VincentRK) July 20, 2020
In the rest of the conversation, seperate from COVID-19, successful trial results, including for Bayer’s finerenone and Boehringer’s empagliflozin, reaped positive engagement from HCPs. One HCP in the US not only congratulated Bayer for their results but also two of the trialists involved in the study. Another US HCP shared Boehringer’s trial results adding his highlights from the report.
More great news for people with diabetic kidney disease: finerenone successful at preventing main renal and CV outcomes in the #FIDELIO trial. Congratulations to @AgarwalRajivMD and @BakrisGeorge as well as @Bayer ! https://t.co/f0J7pqKZQ4 pic.twitter.com/NPfZrkfHqg
— Vlado Perkovic (@VladoPerkovic) July 9, 2020
⚠️⚠️#EMPERORreduced trial via @Boehringer⤵️
📍 Empagliflozin meets primary endpoint in reducing risk of cardiovascular⚰️ or heart failure 🏨in Phase III trial in adults with and without diabetes@secardiologia @redGDPS @SEDiabetes @SociedadSeedo
📂https://t.co/R0RrhSlaDP pic.twitter.com/uMwZvUUF9W— Alfonso Valle (@ValleAlfonso) July 30, 2020
CREATION.co continues to analyse online HCP conversation on a variety of topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
- Data for this research was analysed using CREATION Pinpoint® from the online Twitter conversations of HCPs around the world in English language (other languages are available), between July 1st – July 31st, 2020.
- Vaccines tracked were the 26 COVID-19 vaccine candidates in clinical evaluation before 3 August 2020 update of the WHO Draft landscape of COVID-19 candidate vaccines.
 By Laura McIntyre
By Laura McIntyre 
