Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout July 2021 we tracked the global conversations of over 3,000 HCPs who posted more than 4,800 English-language Twitter posts in relation to product approvals.
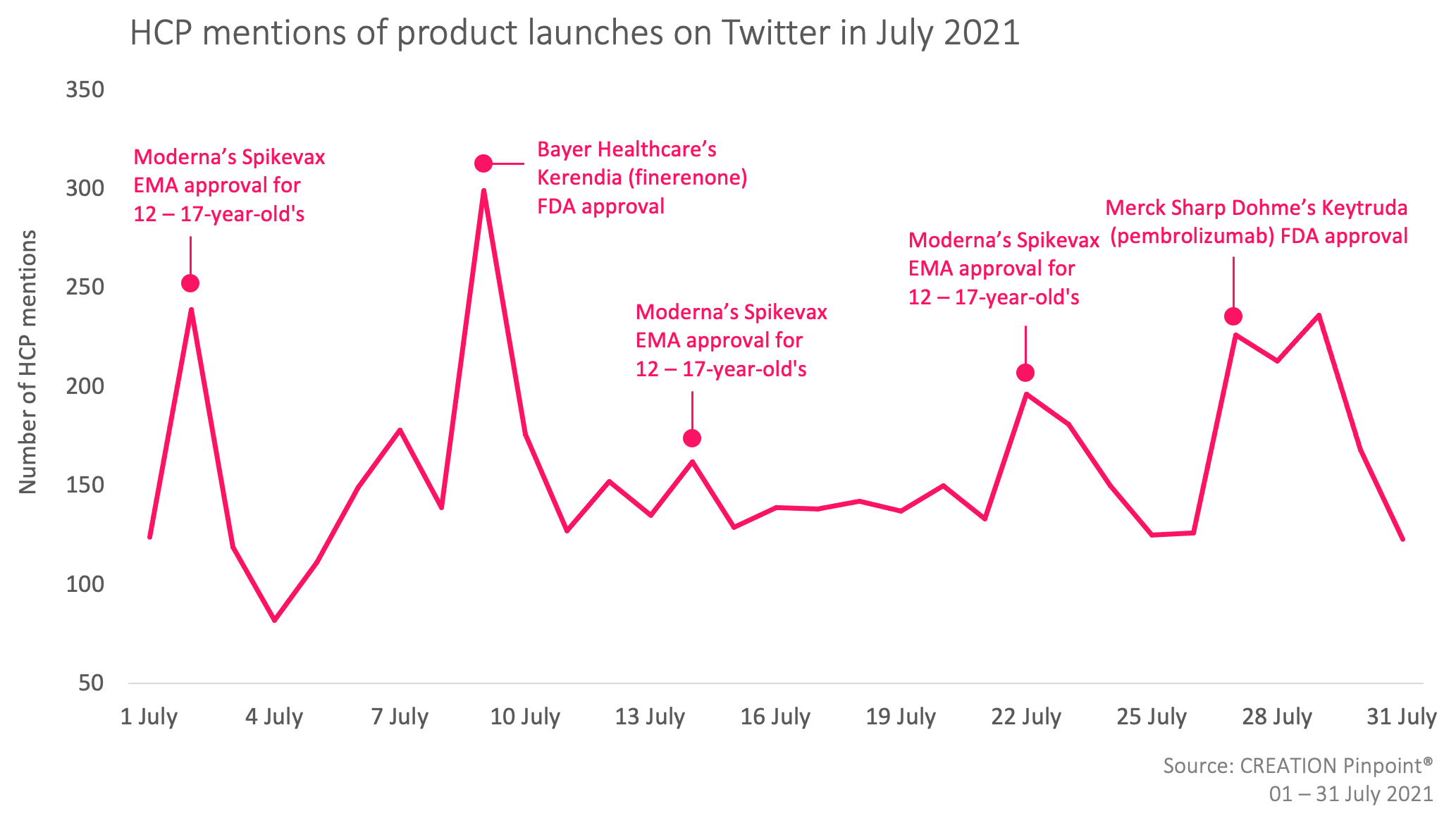
In July 2021, the new product approvals that caught HCPs’ attention were Merck & Co’s KEYTRUDA® (pembrolizumab), triple negative breast cancer treatment (TNBC) treatment, Bayer Healthcare’s Kerendia (finerenone) treatment to reduce the risk of eGFR decline, end stage kidney disease, cardiovascular death & non-fatal myocardial infarction in adult patients with chronic kidney disease (CKD) with type 2 diabetes (T2D), and Moderna’s Spikevax, COVID-19 vaccine for children from the ages of 12.
Several HCPs, such as Medical Oncologist and Hematologist, Dr Hope Rugo, celebrated the FDA approval of KEYTRUDA® (pembrolizumab) for patients with high-risk early-stage triple negative breast cancer (TNBC), stating that it was a “big step for patients with TNBC” and “great news for [their] patients”.
Yay! A big step fwd for pts with TNBC @oncoalert. FDA Approves KEYTRUDA® (pembrolizumab) for Treatment of Patients With High-Risk Early-Stage TNBC With Chemo as Neoadjuvant Treatment, Thenas Single Agent as Adjuvant Treatment After Surgery – Merck. https://t.co/5uoFXPhHTS
— Hope Rugo (@hoperugo) July 27, 2021
Some HCPs, such as, Nephrologists, Dr Mario AL S and Dr Swapnil Hiremath shared their positive surprise towards Kerendia’s (finerenone) “quick” FDA approval, while over 100 HCPs shared the EMA’s post announcing it’s approval of Spikevax to children from the ages of 12 in Europe.
Whoah that happened quickly
Finerenone approved ✅https://t.co/U3hsOHI4wA
Kerendia really though?
Check out our Fidelio discussion and podcast at link: https://t.co/0rexCAmPSv
My concern remains to beware hyperkalemia
— Swapnil Hiremath @[email protected] (@hswapnil) July 9, 2021
The three most shared links from HCPs discussing product launches in July were:
- A New York Times article explaining why the FDA now needs to fully approve mRNA COVID-19 vaccines
- An FDA article about the Kerendia (finerenone) approval for the reduction of risk to serious kidney and heart complications in adults with chronic kidney disease associated with type 2 diabetes
- An EMA article about the Spikevax approval for children ages 12 and above in Europe
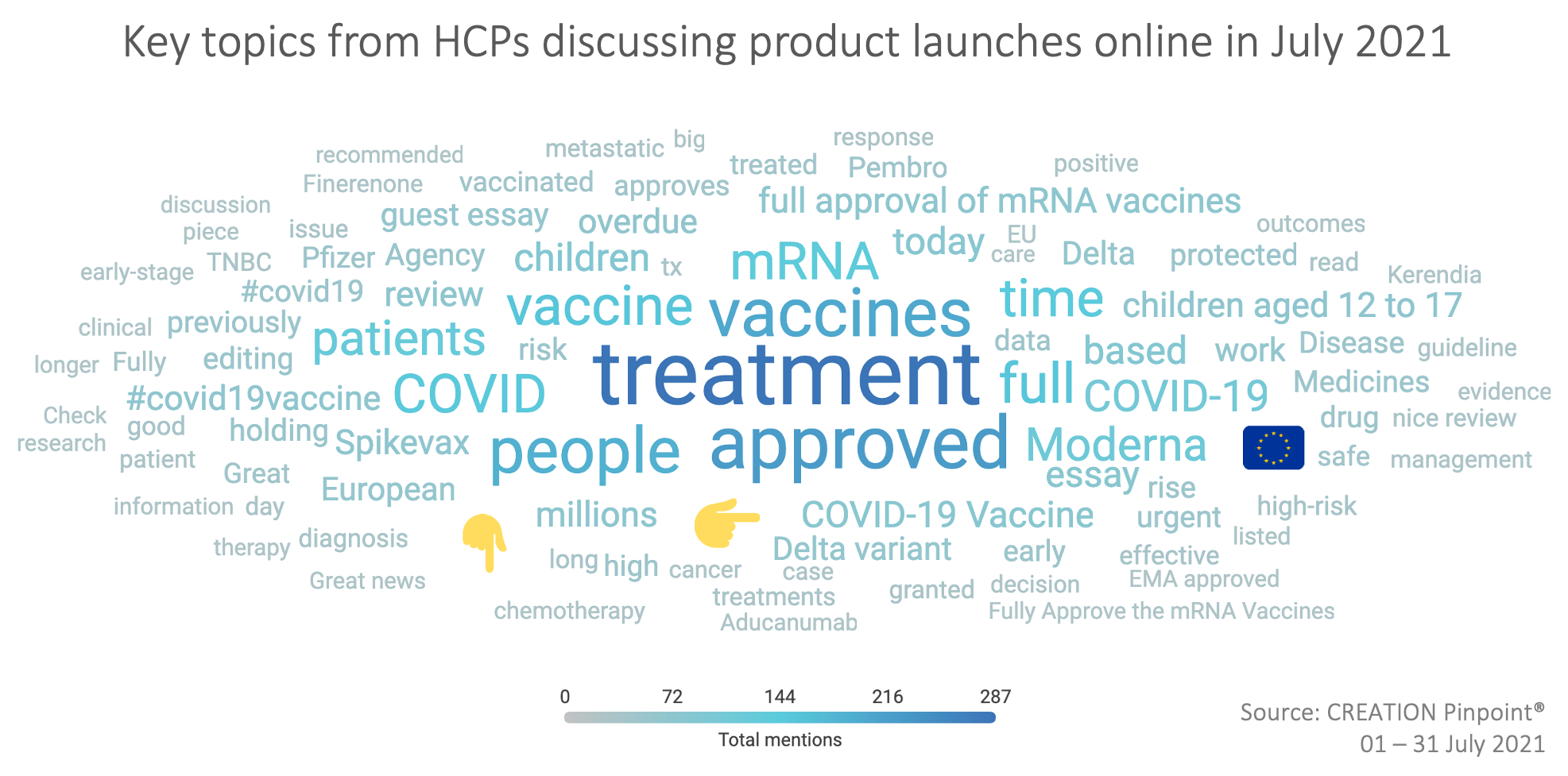
Throughout the month, the EMA’s approval of Spikevax for children aged 12 and above was a key discussion among HCPs, with over 800 HCP mentions of the vaccine.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint®, the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 July and 31 July 2021 were analysed.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 July and 31 July 2021, there were 4,871 HCP mentions of new pharmaceutical product launches and drug approvals from 3,099 unique HCP authors from around the world.
The Product Launch tracker archive


