Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout November 2022 we tracked the global conversations of 1,811 HCPs who posted 2,465 English-language Twitter posts about the launches and approvals of new products.
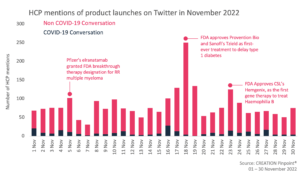
Over the month of November, HCPs discussed and spread the news of several new product approvals, including a new treatment option for multiple myeloma, a gene therapy treatment for haemophilia B, and most notably, the approval of the first ever treatment to delay the onset of type 1 diabetes.
The news of the FDA approval of Provention Bio and Sanofi’s Tzield (teplizumab-mzwv) gained significant attention from HCPs online, with over 150 HCPs mentioning the new treatment in November. This approval was considered particularly important as it is the first ever treatment to delay the onset of type 1 diabetes. HCPs described the treatment as groundbreaking and highlighted the significance of the product as the 1st drug to alter the course of type 1 diabetes in 100 years; however, there was some concern over the cost of the treatment and that its impact is limited to an average delay of onset of roughly 2 years.
Teplizumab is the first FDA-approved therapy for prevention of type 1 #diabetes.
This is truly groundbreaking and such an unmet need. Let’s hope the real world application helps without harm.https://t.co/NvolCSkNIQ#DiabetesAwarenessMonth— Ruchi Mathur MD (@RuchiMathurMD) November 18, 2022
Good news: 1st drug to alter the course of Type 1 diabetes in 100 years approved by @US_FDA
Bad news: it costs ~$200,000/14-day treatment; average delay of onset is ~2 yearshttps://t.co/bVkvbZzAjB pic.twitter.com/2AAIUazEXU— Eric Topol (@EricTopol) November 18, 2022
Earlier in the month some HCPs had also shared the news of Pfizer’s elranatamab gaining breakthrough therapy designation from the FDA as a treatment for relapsed and refractory multiple myeloma. HCPs congratulated Pfizer on the approval, celebrating it as great news, as well as reflecting on the developments in multiple myeloma treatment in the 21st century. They highlighted that 15 new drugs have been approved in the last 20 years, vastly increasing the number of treatment options available.
Pfizer’s Elranatamab Granted FDA Breakthrough Therapy Designation for Relapsed or Refractory Multiple Myeloma – another great news for the field! Congratulations @pfizer_news @Pfizer_France @COMyCongress @TheIACH https://t.co/WbiTws3aNc
— Mohamad Mohty (@Mohty_EBMT) November 4, 2022
My slides and key takeaways from 2 days of intense discussion at the FDA/IMS meeting on drug development in myeloma. Applies to other cancers as well. #MedTwitter @FDAOncology @Myeloma_Society
1) 15 new drugs approved for myeloma in the last 20 years! Incredible. pic.twitter.com/KVLTvVGLe9
— Vincent Rajkumar (@VincentRK) November 10, 2022
The other approval which gained notable attention from HCPs in November, was the FDA approval of the first gene therapy treatment for patients with haemophilia B. Hemgenix (etranacogene dezaparvovec-drlb), which is licenced by CSL gained attention from HCPs for being an innovative and “paradigm shifting” treatment. Others however expressed surprise at the cost of the treatment, with it also holding claim to being the world’s most expensive treatment.
I knew gene therapy for #hemophilia B was going to be expensive, but I did not anticipate it would be this expensive. $3.5 million per dose! https://t.co/bZgPXpBr18 #haemophilia
— Michael Makris (@ProfMakris) November 23, 2022
Paradigm changing news for Hemophilia!! FDA Approves First Gene Therapy to Treat Adults with Hemophilia B | FDA https://t.co/bFx8HahbPR
— Sanjay Ahuja (@ahujadoc) November 23, 2022
The three most shared links from HCPs discussing product launches in June were:
- The American Diabetes Association’s official statement on the approval of Teplizumab
- An FDA press release on the approval of Tzield (teplizumab-mzwv) injection to delay the onset of stage 3 type 1 diabetes
- An FDA press release on the approval of the first gene therapy to treat adults with Hemophilia B
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 November and 30 November 2022 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 November and 30 November 2022, there were 2,465 HCP mentions of new pharmaceutical product launches and drug approvals from 1,811 unique HCP authors from around the world.
Click here to the read the latest Product Launch Tracker
 By Paul Cranston
By Paul Cranston 
