Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout April 2022 we tracked the global conversations of 1,941 HCPs who posted 2,647 English-language Twitter posts about the launches and approvals of new products.
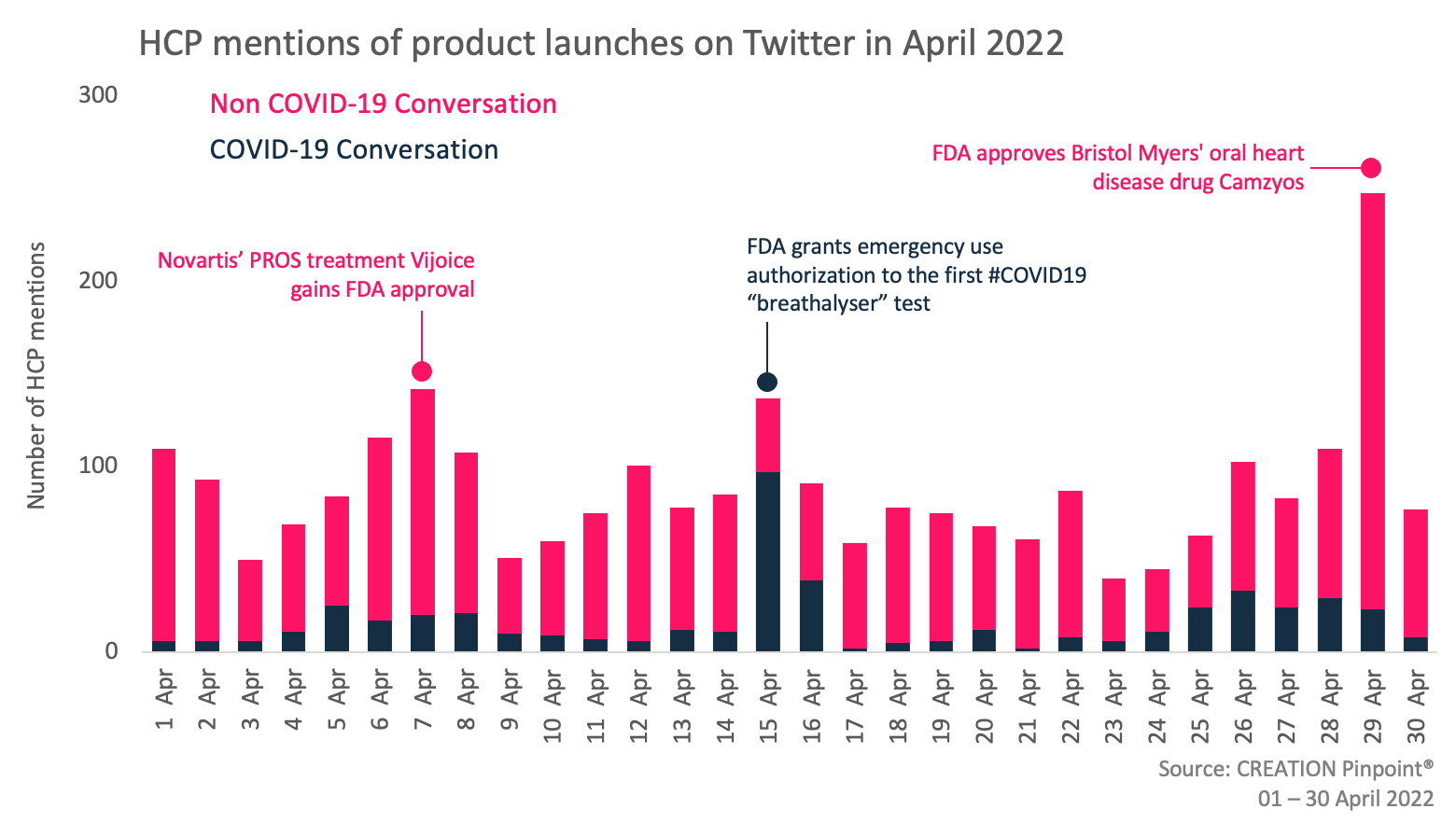
Over the last month, HCPs discussed product approvals for the treatment of a variety of diseases including lymphoma, severe mental illness, and PIK3CA-related overgrowth spectrum. Additionally, in HCPs discussions regarding advances in diagnosing and treating COVID-19, a new ‘breathalyser’ style test for the disease gained attention. However, the approval that generated the most discussion among HCPs in April was a new oral heart disease treatment produced by Bristol Myers Squibb.
Towards the end of April the FDA approved Camzyos (mavacamten) for the treatment of obstructive hypertrophic cardiomyopathy (oHCM). As a first-in-class treatment, many HCPs took to social media to share their positive sentiments towards the Bristol Myers Squibb product, describing it as a big day for the HCM community.
What an awesome day! Mavacamten approved for oHCM Tx today!! Thanks to all the scientists from bench to bedside, trial teams and most of all the HCM patient community! https://t.co/xrvW09Rcos
— Sara Saberi (@S2beri) April 29, 2022
Big day today in the HCM world — FDA just approved first-in-class drug mavacamten for oHCM. Excited to hopefully be one of the 1st in the PNW here @VMFHealth to offer it @purviparwani @selma_carlson @DrBenzigerHeart @gopi_gdanda1 @KateKearney4 @tiffchenMD https://t.co/fpotuDD9gK
— Mariko Harper MD MS (@DrMarikoHarper1) April 29, 2022
Another product to gain approval was BioXcel Therapeutics dexmedetomidine (Iglami) sublingual film for the acute treatment of adult patients with agitation associated with schizophrenia or bipolar I or II disorder in adults. HCPs showed interest in the product, sharing the news of its approval, however some questioned whether agitated patients would be willing to take a sublingual preparation of the drug.
Curious to know how agitated patient with schizophrenia or bipolar get convinced to take this sublingual preparation of dexmedetomidine.
— Om Prakash, MD (@ompsychiatrist) April 7, 2022
HCPs also shared the news of the FDA’s approval of Novartis’ Vijoice (alpelisib) for the treatment of patients with severe manifestations of PIK3CA-related overgrowth spectrum (PROS) who require systematic therapy. Vijoice is the first FDA approved treatment for PROS, a spectrum of rare conditions characterised by overgrowths and blood vessel anomalies affecting the quality of life of patients. HCPs described the approval as huge news for precision medicine
🚨Approval alert 👉🏼Huge news in #PrecisionMedicine 🎯👉🏼FDA approves alpelisib for PIK3CA-related overgrowth spectrum
✅Efficacy based on real-world data a + expanded access program for compassionate use in children+ adults @OncoAlert @tmprowell https://t.co/zIni1VZNE4 pic.twitter.com/Vk4igY6mQD— Vivek Subbiah, MD (@VivekSubbiah) April 6, 2022
In the COVID-19 space, HCP discussion increased as the FDA authorised the emergency use of the InspectIR Breathalyser – the first COVID-19 diagnostic test uses breath samples rather than swab samples to detect the presence of the SARS-CoV 2 virus. When sharing the news of this approval HCPs highlighted its accuracy, which is higher than that of PCR testing, and also suggested it was a good alternative for those who don’t like swabbing their nose.
New—FDA grants emergency use authorization to the first #COVID19 “breathalyzer” test that can detect #SARSCoV2 in a breath sample, within 3 minutes—with 91% of positive samples correctly and 99% of negative samples correctly.https://t.co/2ols6HMC3S
— Eric Feigl-Ding (@DrEricDing) April 15, 2022
Finally, at the beginning of April, the FDA also approved Gilead’s Yescarta (axicabtagene ciloleucel) CAR T-cell therapy for adult patients, as a second-line treatment for adults with large B-cell lymphoma. The news of this approval was met positively by HCPs, with it being described as excellent news for lymphoma patients.
Excellent news for our patients: CAR T-cell therapy (axi-cel) is now approved for patients treated in second line for large B-cell lymphoma. My team at @MDAndersonNews was honored to play a role in the pivotal result. https://t.co/18UJQ77PIL
— Jason Westin, MD FACP (@Lymphoma_Doc) April 1, 2022
The three most shared links from HCPs discussing product launches in April were:
- A New York Times article about the FDA authorisation of a COVID-19 breathalyser test
- An FDA press release of their approval of axicabtagene ciloleucel for second-line treatment of large B-cell lymphoma
- An article by The Verge about the approval of an autonomous X-ray-analyzing AI to be used in the UK
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 April and 30 April 2022 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 April and 30 April 2022, there were 2,647 HCP mentions of new pharmaceutical product launches and drug approvals from 1,941 unique HCP authors from around the world.

 By
Paul Cranston and Tomi Shobande
By
Paul Cranston and Tomi Shobande 



