Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout October 2022 we tracked the global conversations of 1,830 HCPs who posted 2,527 English-language Twitter posts about the launches and approvals of new products.
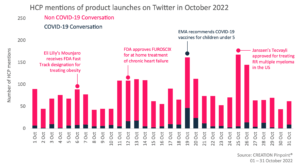
Across the month of October, HCPs commented on and shared the news of many new product approvals across various indications including obesity, multiple myeloma, heart failure and COVID-19.
At the start of the month, HCPs shared the news of Eli Lilly receiving fast track designation for Mounjaro (tirzepatide) for the treatment of adults with obesity. HCPs held the treatment in high regard, claiming the drug to be ‘remarkably effective’ and to have a significant long term benefit to many patients’ cardiometabolic health..
FDA fast tracks #tirzepatide to treat #obesity #overweight Let’s hope insurers cover it. https://t.co/j5Ewboapdt
— dominof (@dominof) October 7, 2022
Eli Lilly’s weight loss drug tirzepatide will surpass the success of AbbVie’s Humira, once it’s FDA approved
1. The drug is remarkably effective
2. The TAM is vast
3. The downstream health effects may significantly prevent cardiometabolic morbidity and mortality— Jared Dashevsky, MD, MEng 💌 (@jareddashevsky) October 10, 2022
HCPs also discussed the FDA’s approval of Janssen’s Tecvayli (teclistimab) for the treatment of adult patients with relapsed or refractory multiple myeloma. The treatment is indicated for patients who received four or more previous lines of therapy. Based on the response rate from a trial, the regulatory agency approved the indication under an accelerated approval process. HCPs declared the approval to be ‘wonderful news’ for patients and an ‘important new option’ for treating multiple myeloma.
Wonderful news for our MM patients. FDA approval for teclistamab.
Grateful to our research study team @StanfordBMT_CT for their hard work and glad to be a part of the phase 2 trial that led to the FDA approval.
Indication ~ to CAR-T, after 4 prior lines of therapy https://t.co/kTLagrdm3E
— Surbhi Sidana, MD (@SurbhiSidanaMD) October 25, 2022
Teclistamab granted accelerated approval for relapsed refractory myeloma in the US, for patients who have received at least 4 prior lines of therapy. This is an important new option for myeloma. https://t.co/dWrHRhN6ch
1/
— Vincent Rajkumar (@VincentRK) October 25, 2022
Another product HCPs discussed online, to gain FDA approval is scPharmaceuticals’ Furoscix, a self-administered subcutaneous injection of furosemide for the treatment of congestion from fluid overload in adults with chronic heart failure. It is the first home treatment which can support patients with worsening congestion who show a decreased responsiveness to other treatments, which usually necessitates hospital admission. Many cardiologists came together to discuss the usefulness of the novel product, with some showing positive sentiments about its potential to keep patients out of hospital, while others were more cautious, primarily due to cost effectiveness and the dose limit of the treatment.
I don’t know how cost effective this will be. It delivers a very small dose after all. Presumably this is for those who l need parentral furosemide for bioavailability/avoiding intestinal oedema etc. Cost/benefit ratio would have been better if it delivered higher doses.
— Mohamed Mohamed, PhD (@dr_mosama) October 13, 2022
In relation to COVID-19, HCPs shared the news of the EMA recommending granting an extension of indication for the COVID-19 vaccine Comirnaty and Spikevax targeting the original strain of SARS-Cov-2. The Committee recommended including the use of in children aged 6 months to 4 years for Comirnaty and use in children aged 6 months to 5 years for Spikevax. Comirnaty and Spikevax are already approved in both adults and children aged from 5 and 6 years, respectively. HCPs predominantly chose to distribute this news from the EU Medicines agency’s official account rather than individually commenting on the approval.
‼️ EMA has recommended authorising the use of mRNA #COVID19vaccines in younger age groups:
➡️From 6 months to 4 years for Comirnaty
➡️From 6 months to 5 years for Spikevax👉 https://t.co/rW0YQ3s3fr pic.twitter.com/6qjEkRjUcC
— EU Medicines Agency (@EMA_News) October 19, 2022
Shared by 18 HCPs
The three most shared links from HCPs discussing product launches in June were:
- An FDA publication about the approval of tecistimab for the treatment of relaxed or refractory multiple myeloma
- A eMPR article about the FDA approval of Furoscix for the treatment of chronic heart failure outpatients
- An Eli Lilly news release announcing the FDA Fast Track designation for tirzepatide for the treatment of adults with obesity
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 October and 31 October 2022 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 October and 31 October 2022, there were 2,527 HCP mentions of new pharmaceutical product launches and drug approvals from 1,830 unique HCP authors from around the world.
Click here to read the latest article

 By
Paul Cranston and Tomi Shobande
By
Paul Cranston and Tomi Shobande 


