Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout November 2021 we tracked the global conversations of 3,037 HCPs who posted 4,615 English-language Twitter posts about the launches and approvals of new products.
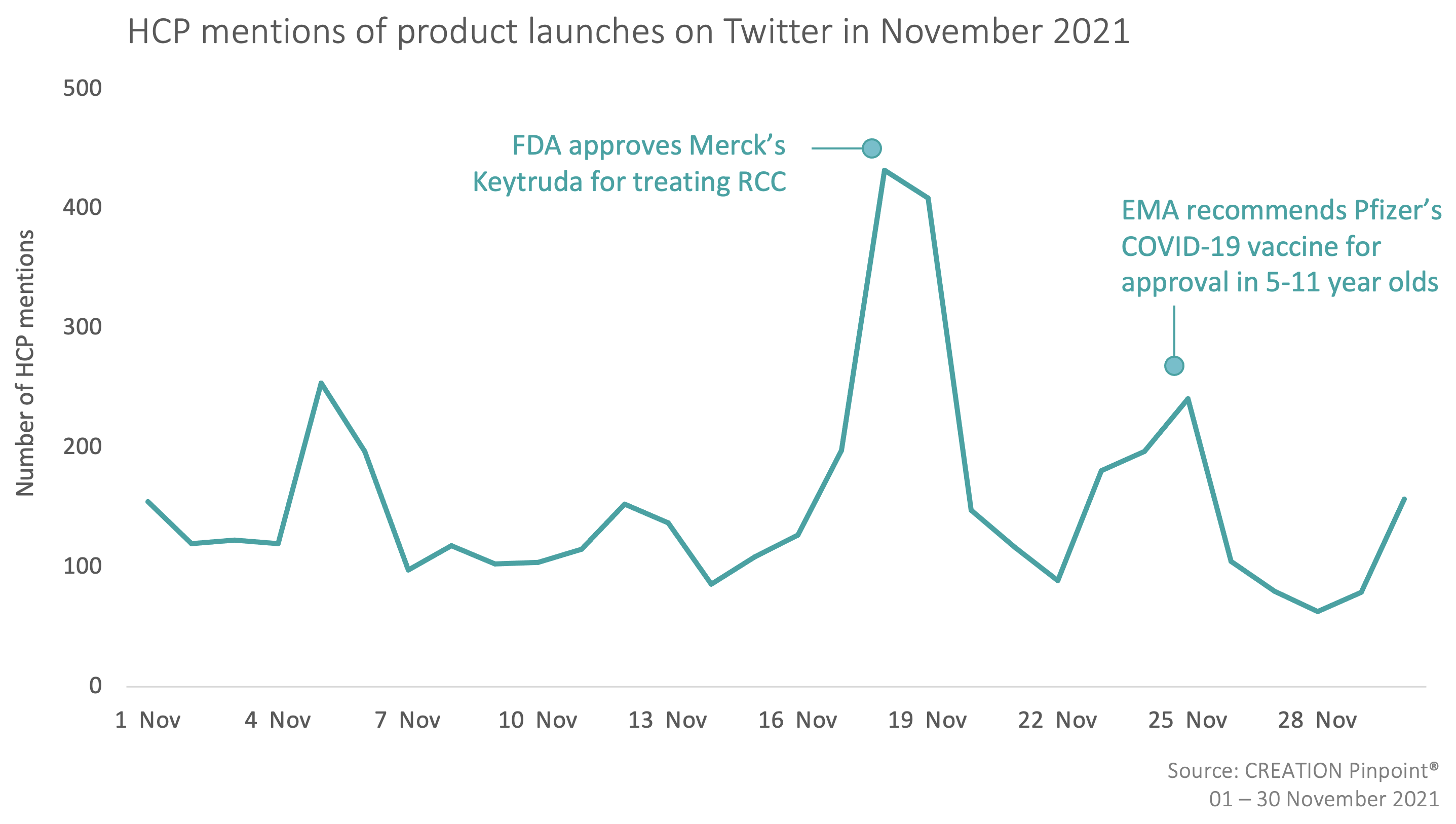
Over the course of November, the FDA approval of Merck MSD’s Keytruda (pembrolizumab) for the adjuvant treatment of patients with renal cell carcinoma (RCC) was the key talking point among HCPs. The treatment is to be used in patients at intermediate-high or high risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions. HCPs were pleased with the news as they saw it as a step in the right direction and a game-changer for the kidney cancer therapy area.
adjuvant pembrolizumab is FDA approved in int/high RCC (post nephrectomy) with significant DFS data. While OS is not yet mature it is trending in the right direction making this approach attractive. ESMO guidelines are also supportive (level 1C). https://t.co/ALf634pN5r
— Tom Powles (@tompowles1) November 18, 2021
Landmark day in kidney cancer history. Adjuvant pembrolizumab now FDA approved in RCC at high risk of relapse post nephrectomy. Game changer. Congrats @DrChoueiri @tompowles1 https://t.co/od86pAAzkM
— Rana McKay (@DrRanaMcKay) November 18, 2021
The FDA also approved Takeda’s Livtencity (maribavir) as the first and only treatment for people aged 12 and older with post-transplant cytomegalovirus (CMV) that is refractory, with or without genotypic resistance, to conventional antiviral therapies. HCPs shared their excitement for the approval of Livtencity (maribavir) and look forward to more treatment options being available.
A new drug for CMV tx? Say it ain’t so!! Maribavir (Livtencity) is a welcome option. Feels like the holidays have arrived. 🎄💊🤓 Now we just need ceftolozane/tazobactam to return… @SIDPharm @accpinfdprn #idgeeks https://t.co/L3vczbzmHs
— Julie Ann Justo (@julie_justo) November 24, 2021
Big deal for #BMT patients – @US_FDA approves maribavir for treatment of resistant post-transplant CMV infection #bmtsm https://t.co/pgYZ9rJUaE
— Navneet Majhail, MD, MS (@BldCancerDoc) November 24, 2021
HCPs also welcomed the news that the European Medicines Agency (EMA) recommended Pfizer’s COVID-19 Vaccine for approval in children aged 5-11. This age group would receive a lower dose of the Comirnaty vaccine than used in people aged 12 and older.
Comirnaty COVID-19 vaccine: EMA recommends approval for children aged 5 to 11 | European Medicines Agency 👏👏👏#vaccination #covid19 #children 👧💉 https://t.co/juPU8K1xY5
— María (Maria del Mar) Tomas (@MariadelMarTom) November 25, 2021
Wonderful news! EMA has recommended approval of Pfizer vaccine for 5 to 11 year olds #vaccineswork https://t.co/uOpDzQzqcg
— Christine Loscher (@celoscher) November 25, 2021
The three most shared links from HCPs discussing product launches in November were:
- An FDA article about the approval of pembrolizumab for adjuvant treatment of renal cell carcinoma.
- An EMA article about the EMA recommending the comirnaty COVID-19 vaccine for approval for children aged 5 to 11
- An FDA article about the FDA Expanding Eligibility for COVID-19 Vaccine Boosters
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint®, the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 November and 30 November 2021 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 November and 30 November 2021, there were 4,615 HCP mentions of new pharmaceutical product launches and drug approvals from 3,037 unique HCP authors from around the world.

 By
Paul Cranston and Tomi Shobande
By
Paul Cranston and Tomi Shobande 

