Discover what healthcare professionals (HCPs) think about pharmaceutical products and their manufacturers, as it happens, through CREATION.co’s tracking updates.
How are HCPs responding to drug launch news? How are HCPs talking about or engaging with the Top 50 pharmaceutical companies on social media? Each week CREATION.co’s tracking updates bring you the latest insights from the conversation of HCPs across the globe discussing these topics and more.
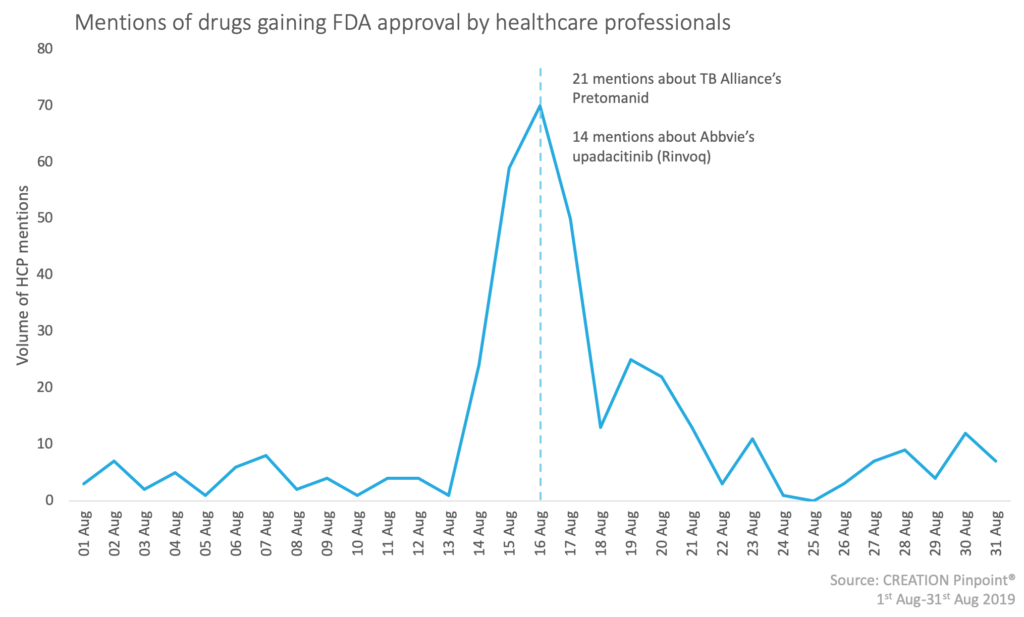
CREATION Pinpoint’s Drug Launch Tracker shows how healthcare professionals (HCP) respond to drug launch news, as it happens.
Over the month of August there have been more than 200 different drug submissions that gained FDA approval in various therapy areas but two particularly caught the attention of healthcare professionals. During the month, 334 HCPs posted 384 tweets showing their excitement for new treatments and the anticipation of the effect that they will have on their patients’ quality of life.
One of the drugs that sparked the interest of HCPs this month was Abbvie’s oral JAK inhibitor Rinvoq (upadacitinib). This drug has been approved for the treatment of moderate to severe rheumatoid arthritis. HCPs shared the news but with some concerns over the safety and price of the product. Three HCPs posted an article by BioPharma Dive which outlined the difficulties that doctors have had with insurance companies when prescribing JAK inhibitors. The safety concern expressed related to drug interaction; Dr John Cush pointed out that Rinvoq cannot be combined with other JAKs or biologics as this has the possibility of causing blood clots.
HCPs talked most about the FDA approval of TB Alliance’s Pretomanid tablets in combination with bedaquiline and linezolid to treat highly treatment-resistant tuberculosis (TB) of the lungs. This new breakthrough was welcomed by HCPs as well as other health policy influencers who pointed out that multidrug-resistant tuberculosis is an enormous public health threat. HCPs noted that, even though this condition is one of the most deadly infectious diseases, this research was being funded by a non-profit organisation.
Most influential author

Dr John Cush had the greatest level of influence on the online conversations of other HCPs talking about drug launches in August with four original posts that were retweeted 27 times by his peers on average reaching around 12,000 different HCPs.
I am going to be tracking FDA approvals each month to keep you up-to-date with what HCPs think about new products. You can follow this tracking here at CREATION Knowledge.


