In this article, we look at:
- The role of social media in drug safety public health information.
- How much online drug conversation relates to side effects.
- Uncovering real-world cases and how drugs are being used.
- Discovering little-known side effects.
Around one in seven people suffer from migraines, making it the third most common disease in the world. Triptans, a group of 5-HT receptor agonists, have been widely and successfully used for the acute treatment of migraines since the 1990s. In December 2019, gepants, a new class of migraine treatment, entered the market with Allergan’s Ubrelvy, the first CGRP receptor antagonist to gain FDA approval. The new class has been shown to be safe and effective with the added benefit of use by people with vascular disease, heart disease and stroke.
With clinical trials positioning gepants as a suitable alternative to triptans, the established drug class, we wanted to understand the patient experience with, and safety of, Allergan’s new offering by studying online conversations.
High level of discussion about drug side effects online
Our investigation led us to observe HCP, patient and public conversations worldwide relating to migraine drugs on all open social media platforms between 1st January 2020 and 31st March 2021. We identified and collected conversations mentioning Ubrelvy, a triptan or an unspecified migraine drug. From this dataset we drilled-down further into conversations relating to common side-effects, as well as less well known side effects identified through exploratory conversation analysis*.
Across the seven triptans, Ubrelvy and conversations that referred to a migraine drug without specifying a particular product, side effects made up 22% of the conversation. Axert received the lowest total number of posts (492) but the highest percentage of side effect mentions (40%).
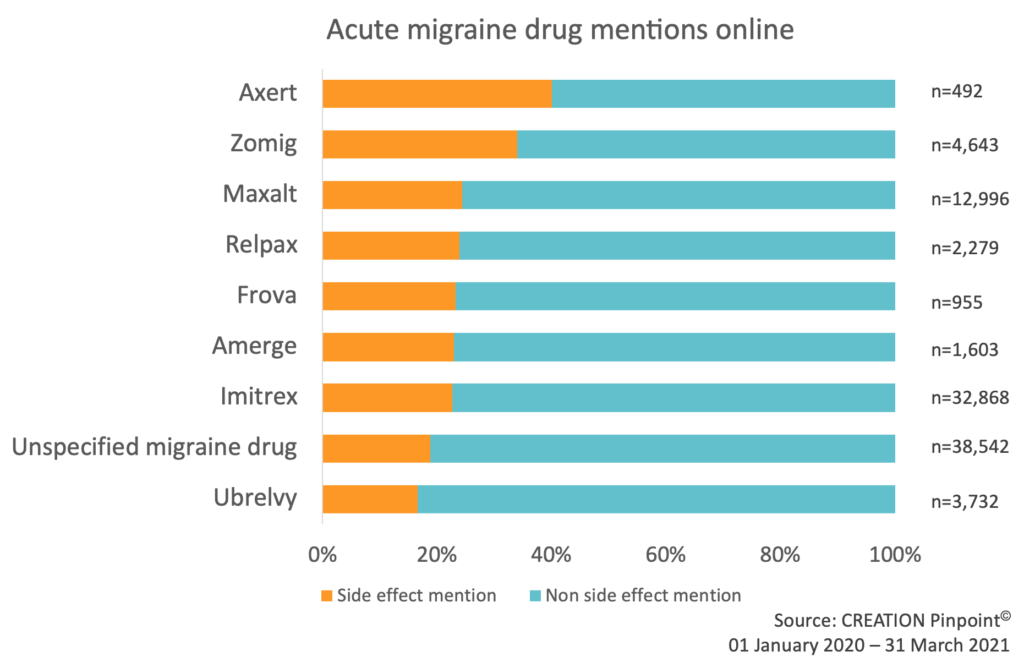
Figure 1: Percentage of migraine drug conversations that mention a side effect and do not mention a side effect. Data includes conversations from HCPs, patients and the public on open social media sites.
From previous social media research, CREATION.co has investigated product conversation topics and developed insight into HCP and patient opinions and sentiment towards treatment, including safety and efficacy data and use in clinical practice. Establishing the level of conversation around side effects is an essential tool in understanding product perception and from this framework we set out to accurately isolate the voice of individuals who have actually taken a product from those simply talking about the drug. These are the people whose experiences are going to teach us about the safety profile of a drug and could provide early drug safety signals to drug manufacturers.
Identifying patient cases
Using CREATION Pinpoint© we were able to distill individual patient experiences from the noise of wider side effect conversation. These patient experiences were primarily being reported on Twitter and Reddit, with a number of other blogging and health platforms being used.
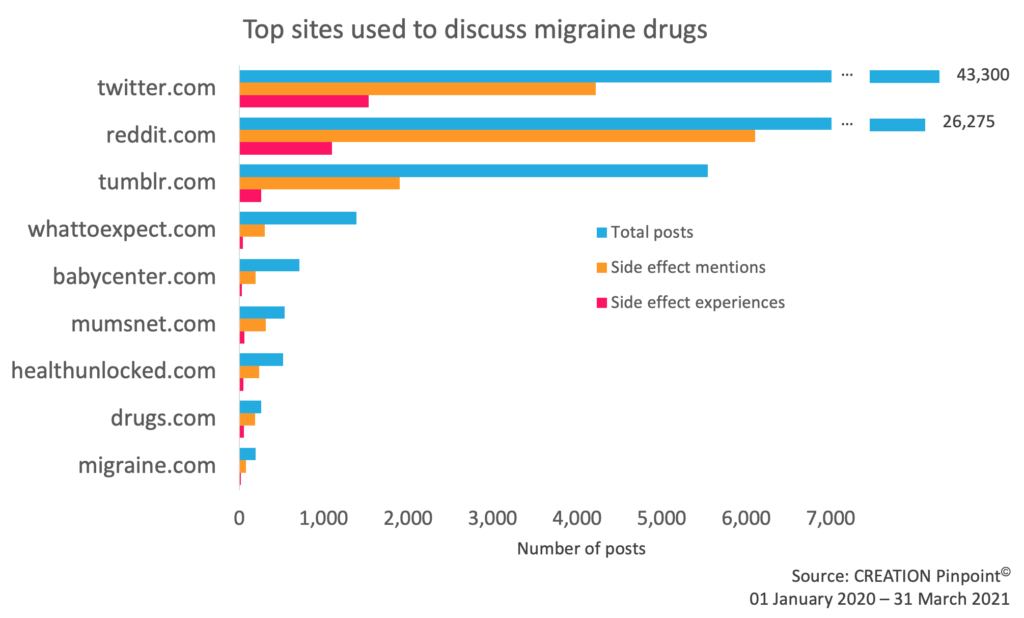
Figure 2: Social media platforms used to discuss migraine drugs. Twitter was the most common platform for mentioning migraine drugs but side effects were more often mentioned on Reddit. Patients shared their experiences with migraine drugs mostly on Twitter and Reddit.
We found that lived patient experiences made up around 17% of the total mentions about migraine drug side effects from HCPs, patients and the public. Once we had filtered out side effect conversations which did not contain patient experiences, we observed that nausea was most commonly reported by patients. The finding that a commonly experienced side effect was commonly reported online may in itself be unremarkable; however, by digging a little deeper we find a host of other events of varying degrees of severity. Many patients look for advice and reassurance from others who may be experiencing the same issues.
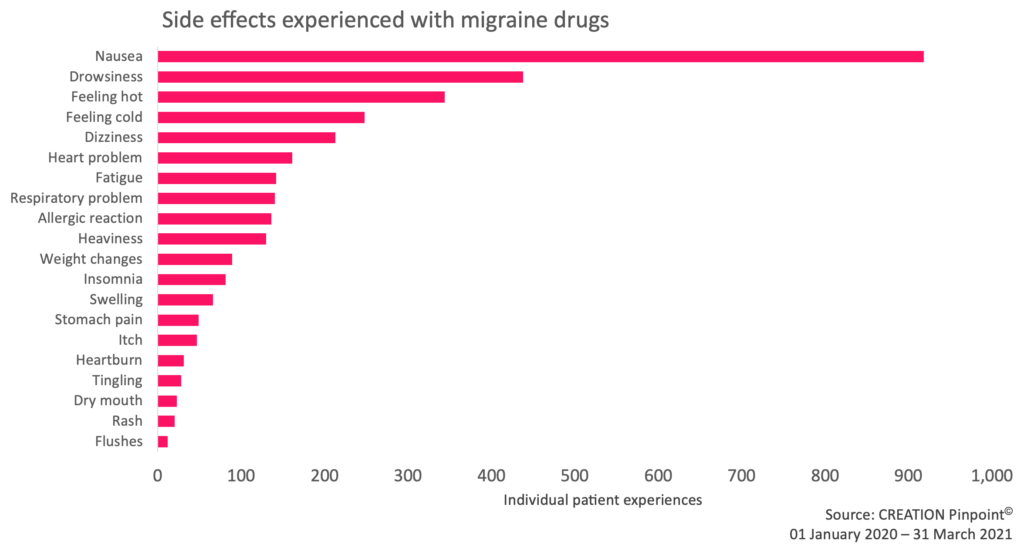
Figure 3: Drug side effects experienced by migraine patients, as expressed on social media. Migraine drugs refer to Ubrelvy, a triptan or an unspecified migraine drug.
Comment
byu/Ninotchk from discussion
inmigraine
Discovering uncommon side effects
Beyond nausea and other common side effects, we found that we could identify patient conversations that expressed side effects rarely mentioned on patient information websites. For instance, we identified 81 patient cases of insomnia – more than the number of patients reporting to have experienced commonly listed side effects such as itches, tingling, rashes and swelling.
“Beyond nausea and other common side effects, we found that we could identify patient conversations that expressed side effects rarely mentioned on patient information websites.”
Adam Doggett
Senior Data Scientist, CREATION.co
Whilst drowsiness is commonly listed on patient information sites and was the second most reported side effect online with 438 patient cases, insomnia, as a rarely acknowledged side effect, denotes an underrepresented group when it comes to medical information online. Some patients, such as Reddit user ‘mamawhalejg’, expressed difficulties finding relevant information and such a lack of guidance online is prompting them to seek reassurance that they are not alone.
That insomnia was mentioned in association with the first-in-class gepant may point toward an issue which goes beyond the lack of information about migraine treatments and insomnia and perhaps suggests that this patient experience is not well recognised by the medical community.
Investigating reporting frequencies
Reddit user RedOakMountain’s interaction with a nurse about the relative occurrence of insomnia with Ubrelvy is insightful and leads us to consider whether more people experience insomnia than is typically accepted by the medical community.
Comment
byu/mamawhalejg from discussion
incgrpMigraine
To further investigate possible underrepresentation of Ubrelvy insomnia information online, we calculated proportional reporting ratios (PRR), which indicates how frequently each side effect is reported in relation to Ubrelvy, compared to how frequently each side effect is reported by patients taking a different migraine drug. We compared the relative reporting frequencies of drug event pairs using the triptan class as comparator.
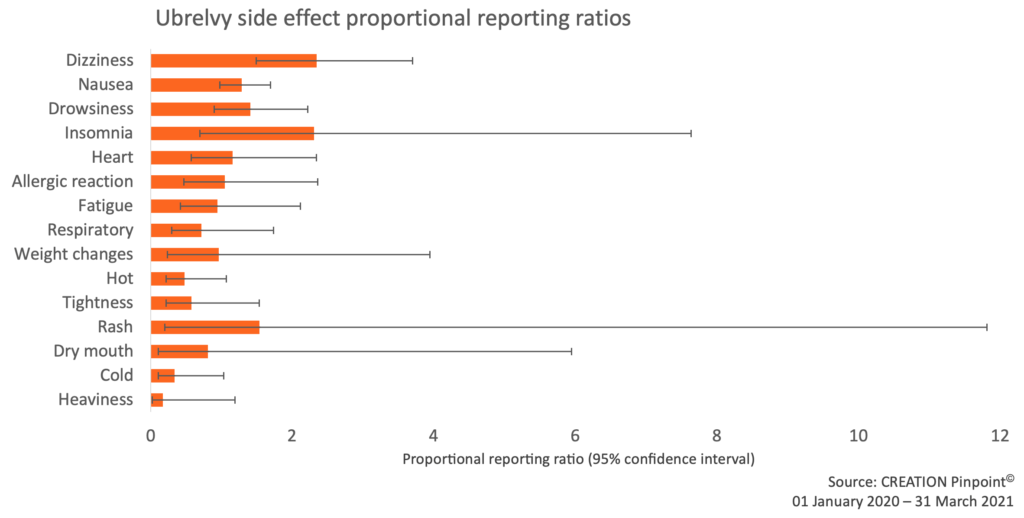
Figure 4: Proportional reporting ratios of Ubrelvy side effects using the Triptans class as comparator. Insomnia PRR = 2.3 (0.7 – 7.6).
A PRR of 2.3 indicates that for the insomnia-Ubrelvy pair, the probability of reporting insomnia with Ubrelvy rather than another side effect is 2.3 times higher compared to the probability for triptans. The matter is complicated by the wide confidence intervals (in 95% of cases the true PRR will fall between 0.7 and 7.6) bringing a degree of uncertainty into any conclusion. However, that experiences of insomnia are being reported online more than expected suggests that further investigation would be worthwhile.
Beyond side effects: product complaints, drug interactions and other events
Our exploration into migraine drug side-effect patient cases is just the tip of the iceberg. The opportunity to understand the profile, experience and safety of a drug goes beyond the elements of pharmacovigilance investigated here. In fact, our research uncovered a number of other highly important and relevant conversations around drug safety including package complaints, potential drug interactions, off-label and drug use during pregnancy.
Comment
byu/obviousocean from discussion
inmigraine
Who ever designed the packaging for migraine meds deserves a kick in the ass!
byu/AnandaLai77 inmigraine
Comment
byu/AnandaLai77 from discussion
inmigraine
Social media has huge potential for collecting real-world evidence for drug safety monitoring
Social media has shown itself to be an extremely valuable tool for understanding the needs and behaviours of groups, and there is huge potential for collecting real-world evidence for drug safety monitoring and beyond. From lesser known side effects and drug use in particular patient groups to off label use and drug interaction, social media is providing the resources required to gain drug safety profile insight, inform product decisions and improve patient safety and experiences.
To keep up to date with the latest advancements in using social media analysis to uncover new insights, sign up to our monthly eJournal or get in touch, we’d love to help.
Note
- *For more information on PV reporting requirements for this type of research, please get in touch or read our Pharmacovigilance in HCP social media listening white paper.
 By Adam Doggett
By Adam Doggett 


