Discover what healthcare professionals (HCPs) think about pharmaceutical products and their manufacturers, as it happens, through CREATION.co’s tracking updates.
How are HCPs responding to drug launch news? How are HCPs talking about or engaging with the Top 50 pharmaceutical companies on social media? Each week CREATION.co’s tracking updates bring you the latest insights from the conversation of HCPs across the globe discussing these topics and more.
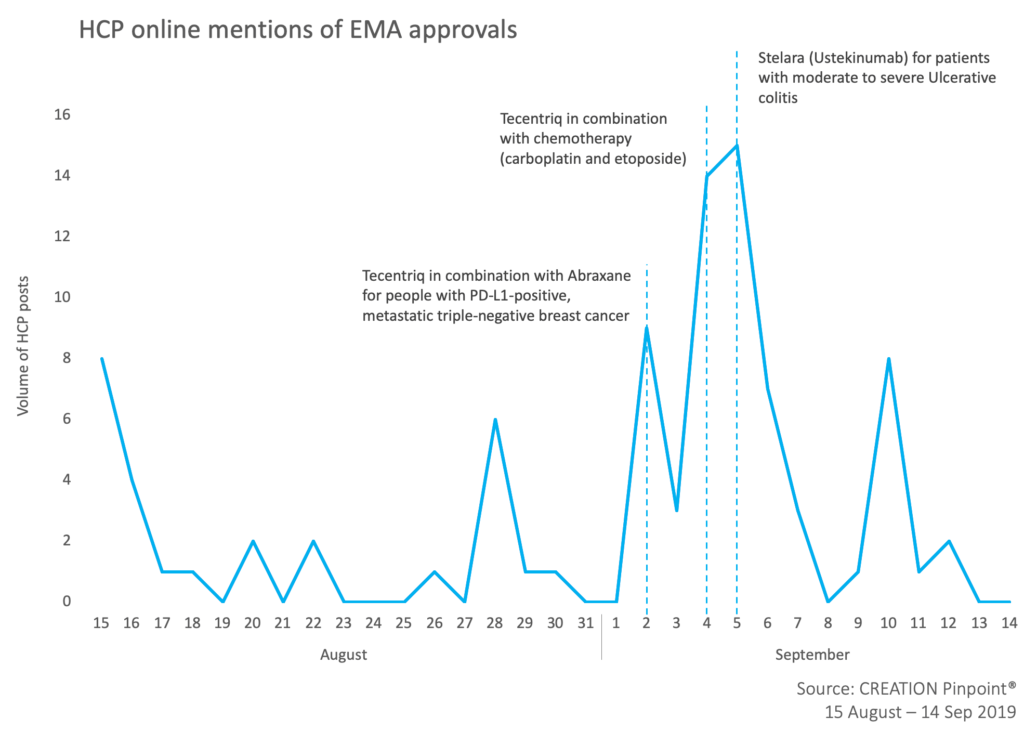
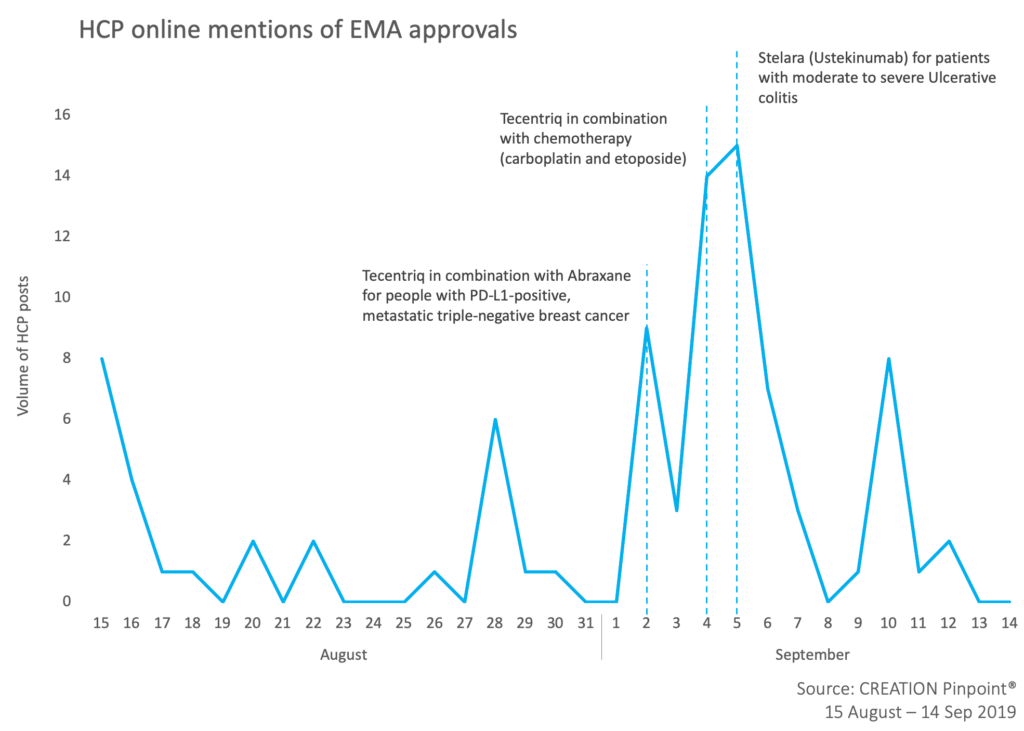
Over the last four weeks HCPs shared news of recently approved medicines in Europe. The most discussed approval among HCPs online was Janssen’s Stelara (ustekinumab) expansion of use for the treatment of moderately to severely active ulcerative colitis in adults failing previous therapies.
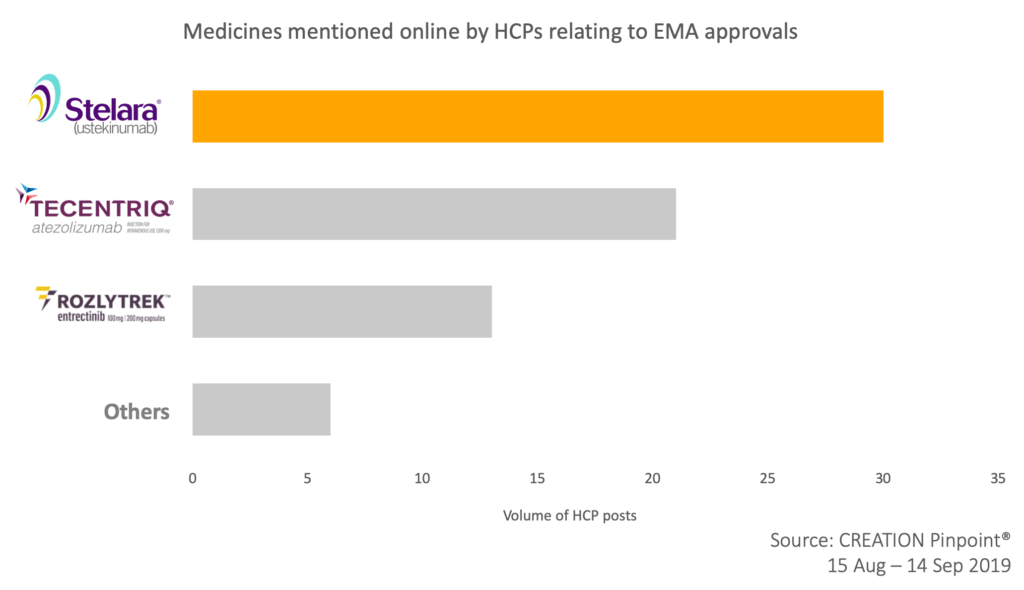
The product was mentioned 30 times with HCPs regarding its approval as “great news to the patients and their doctors” and expressing expectations to soon see this label expansion in the United States.
Not far behind with 21 product mention was Roche’s Tecentriq (atelizolumab) which was granted two approvals in the oncology space – combination with chemotherapy in PD-L1-positive, metastatic triple-negative breast cancer and extensive-stage small cell lung cancer. HCPs positively shared the news, some hoping that Tecentriq changes the standard of care in SCLC.
Meanwhile, the FDA approval of another Roche drug, Rozlytrek (entrectinib), caught HCP attention in a different way. A post shared by a medical oncologist in Spain was retweeted by 13 HCPs globally in which he highlighted EMA approval as a possible challenge for entrectinib. This raises the question: what impact do FDA decisions have on EMA and vice versa?
We are tracking the HCP reaction to EMA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates, including drug approvals, within the Tracking section of CREATION Knowledge.
Methodology notes:
- Data for this research was analysed from the online Twitter conversations of HCPs in English language only (all other languages are available), between Aug 15th and Sep 14th 2019
 By Laura McIntyre
By Laura McIntyre