In brief:
- Last week, Pfizer and BioNTech announce vaccine candidate.
- UK HCPs are pleased with it’s 90% effectiveness.
- However key concerns are consistently raised over storage and administration.
- Highlighting the importance of understanding what your HCP audience are saying in this fast-changing landscape.
- Keep up to date with the online HCP conversation relating to COVID-19 vaccines with the regular COVID-19 vaccine tracker.
The ongoing fight against COVID-19 has seen many companies in the pharmaceutical industry striving to develop an effective vaccine against the virus. One such effort comes from a collaboration between Pfizer and BioNTech, who announced the promising efficacy of their vaccine candidate in a press release on Monday 9th November 2020. BNT162b2 was found to be more than 90% effective in preventing COVID-19 in participants, in the first interim analysis of its global phase 3 study.
This news brings much hope to the public, with the UK government announcing that it has already procured 40 million doses of the vaccine, and stating that the NHS is ready to begin a vaccination programme in the most vulnerable once fully approved by the JCVI (Joint Committee on Vaccination and Immunisation). Within the first week after the announcement, over 2,000 UK healthcare professionals (HCPs) have offered their reactions on social media.
Amongst many posts celebrating the success of the vaccine, three key concerns were raised by UK HCPs in response to Pfizer’s vaccine announcement, which are displayed in the chart below.
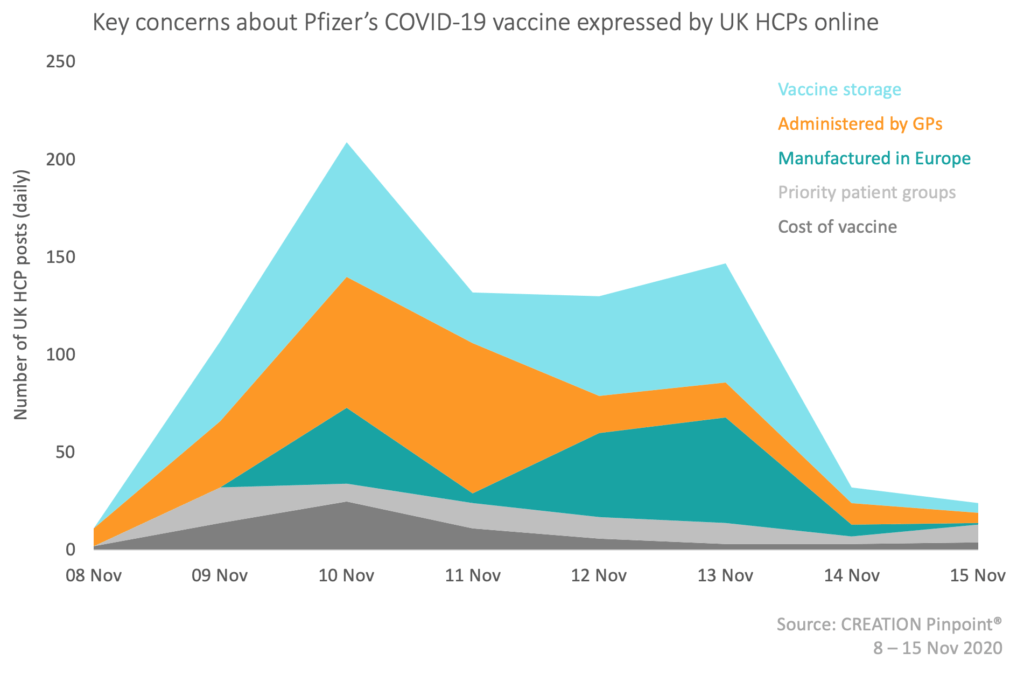
Vaccine storage
The concern most frequently discussed by UK HCPs during this time was the logistical challenge of storing the vaccine, which must be kept at -70/-80 C until ready for use in patients. Those working in GP surgeries and hospitals say they do not currently have appropriate facilities to make this achievable. Others noted that this is even more of a challenge in developing nations, meaning that the vaccine is unlikely to be accessible in much of the world.
Covid vaccine: First 'milestone' vaccine offers 90% protection. This is very welcome news and a credit to science though there are logistical problems as the vaccine has to be stored at -80C and still unsure how long immunity lasts for. https://t.co/1FcQWcOmxV
— Onkar Sahota AM (@DrOnkarSahotaAM) November 9, 2020
Administered by GPs
Another topic of unease for UK HCPs is the role that GPs are expected to play in administering the vaccine directly to patients. The requirements laid out by the UK government, including delivering the vaccine 7 days a week, have been described as “completely mad” by UK HCPs. Many are worried about what the implications of this will be for non COVID-19 appointments, after being told to “scale back care”. Other HCPs say that the responsibility for vaccine administration should be given to nurses or technicians, so that GPs can continue to see patients in a normal capacity.
The conditions under which GPs have been asked to deliver the vaccine seem completely mad
Can @MattHancock or anyone explain to me how they are supposed to:
🟢 Deliver them 7 days a week 12 hrs a day with no extra staff?
🟢 Not get paid for 1 jab?
🟢 Monitor all for 15 mins?— Roshana 🦴 (@RoshanaMN) November 10, 2020
Manufactured in Europe
HCPs have also expressed uncertainties about the practicalities of accessing Pfizer’s vaccine in the UK. As the vaccine is being manufactured in Belgium and Germany, there are concerns not only around the cold chain transportation of the vaccine across countries, but also concerns about the impact that Brexit may have upon free movement of goods into UK borders.
If approved, the Pfizer BioNTech vaccine will be manufactured in Germany and Belgium. We have ordered 40 million doses. Their transport is temperature and time critical. Let’s hope there are no issues that would prevent free and fast movement of goods at our borders in January.
— Dr Phil Hammond 💙 (@drphilhammond) November 12, 2020
As the world awaits the first COVID-19 vaccine approval, and with more and more news every day around the different vaccine candidates, CREATION.CO are committed to tracking HCP reactions as the landscape develops. Keep up to date with the online HCP conversation relating to COVID-19 vaccines with our monthly COVID-19 vaccine tracker.
This article studied 5,212 posts from 2,344 UK HCP authors on social media from 8 – 15 November 2020. Data includes posts which specifically mention the Pfizer/BioNTech vaccine candidate as well as posts discussing COVID-19 vaccines generically.

 By Laura Marsh
By Laura Marsh