Does ambiguous lexicon hinder rare disease treatment knowledge?
Over recent years as we have tracked the emerging online conversation of healthcare professionals (HCPs), physicians have been highly engaged in conversations about new treatment options in oncology that promise to increase overall survival rates and patients’ quality of life.
A brief study of online HCP Tweets in the United States about BRCA (breast cancer suppressor genes) mutation, for example, shows peaks in HCP conversations after advances in treatment or testing options.
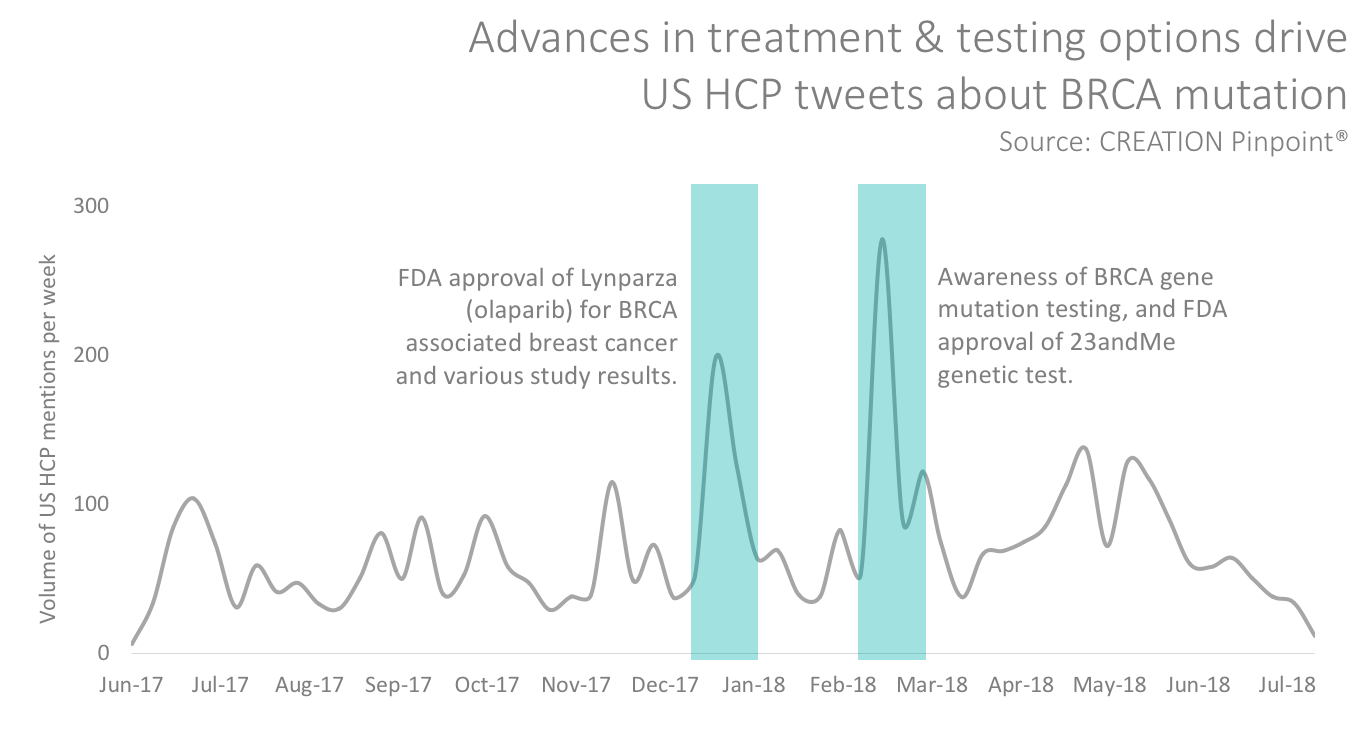
As new treatments for diseases emerge, the collective knowledge shared among HCPs can be powerful for collaborative learning. But in new areas, the specific lexicon used to describe a disease may still be emerging, which may delay the dissemination of information among peers online.
A case in point is the treatment of germline BRCA-mutated breast cancer, which affects patients with inherited mutation of BRCA genes. Analysis of HCP online conversations about the condition reveals a diverse lexicon used when describing similar concepts.
![]()
While the variety of terms used to describe similar or identical indications may prove challenging for HCPs discussing this emerging area of treatment, it should also be an important consideration for treatment manufacturers. Even after the FDA approved AstraZeneca’s product, olaparib, earlier this year for germline BRCA-mutated (gBRCAm), HER2-negative metastatic breast cancer, HCPs used a variety of terms to describe the condition when talking about the FDA approval.
Aditya Bardia, MD, a medical oncologist at Harvard Medical School, mentioned “patients with metastatic breast cancer and germline BRCA mutations” in his Tweet.
FDA approves PARP inhibitor, olaparib/Lynparza for patients with metastatic breast cancer and germline BRCA mutations. #bcsm https://t.co/GUZZ3TOGlm
— Aditya Bardia, MD (@dradityabardia) January 12, 2018
Medical oncologist and clinical trialist Andrea Apolo, MD, who is based at the National Cancer Institute’s Center for Cancer Research, mentioned “BRCA+ Breast Cancer” in her tweet.
FDA Approves Olaparib for BRCA+ Breast Cancer https://t.co/UrXlLyuT7A via @onclive
— Andrea Apolo, M.D. (@apolo_andrea) January 12, 2018
Meanwhile, doctor-journalist Elaine Schattner tweeted about treating “…metastatic breast cancer in patients w/ inherited BRCA mutations”.
https://twitter.com/ESchattner/status/951831026665484288
AstraZeneca’s own US website for the product describes gBRCA-MUTATED, HER2-NEGATIVE METASTATIC BREAST CANCER, reflecting only a slight variation from the specific words used by the FDA. But the full term was not mentioned once by any US HCP over the course of our analysis which included more than 6,000 US HCP tweets related to gBRCA.
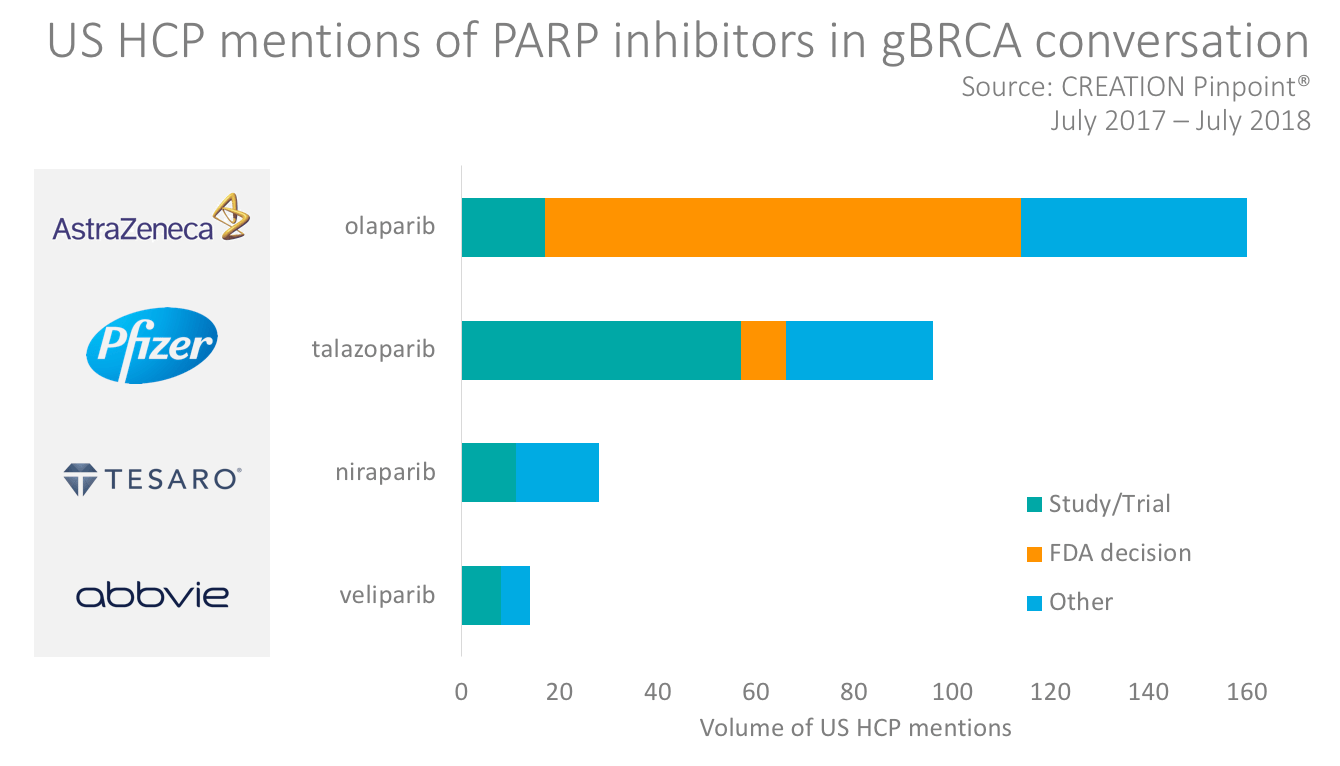
With more treatments emerging, a consistent way of describing the rare cancer condition may help manufacturers and HCPs to connect and communicate more effectively. In Pfizer’s announcement of FDA priority review designation for its product, talazoparib, it used the term germline (inherited) BRCA-mutated (gBRCAm), HER2-negative locally advanced or metastatic breast cancer (MBC). The news of the FDA decision sparked some HCP Tweets about the product but most of the HCP conversation about talazoparib was in response to clinical trial results. None of the HCP tweets included the full name of the indication as described by Pfizer.
Other emerging PARP inhibitors mentioned by HCPs for treating gBRCA breast cancer included Tesaro’s niraparib – already approved for use in ovarian cancer – and Abbvie’s veliparib. Both products were mentioned by HCPs discussing clinical trials, but their reach implies a relatively low level of awareness or interest in the treatments to date compared with Pfizer and AstraZeneca’s products.
Manufacturers engaging HCPs in Breast Cancer
Beyond emerging gBRCA treatments, the breast cancer therapy environment is complex and changing fast. In the wider area of breast cancer, HCPs talk about manufacturers mostly in relation to products, but there are other hot topics that inspired HCPs to talk about companies including pricing, trials and congress activity.
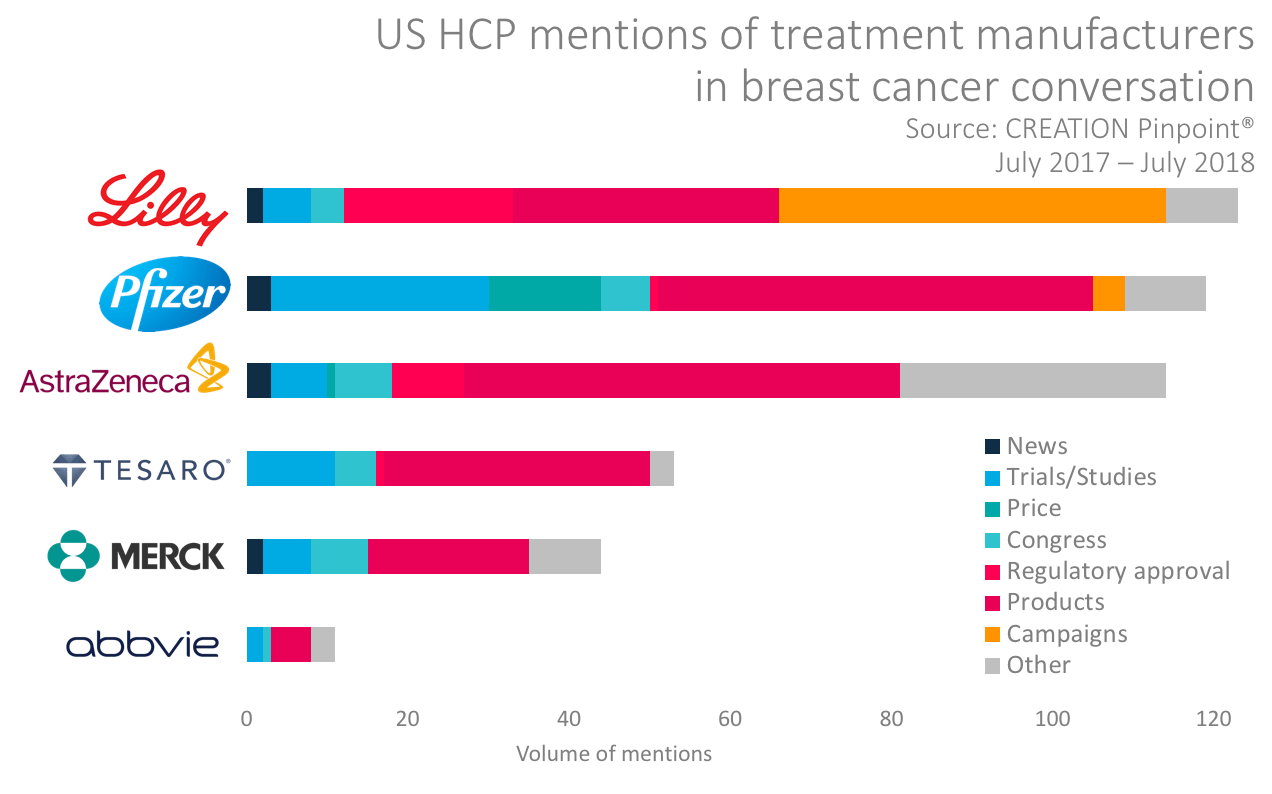
Some manufacturers have launched or supported online advocacy campaigns; Lilly was particularly successful in stimulating HCP posts when it promised to pay $100 for every mention of hashtag #MoreforMBC.
Spectacular message on metastatic #BC. Early detection saves many lives, but not all. More to be done to prevent / treat #MBC. #MoreforMBC https://t.co/r1NwV7vpaH
— Nicole Saphier, MD (@NBSaphierMD) October 25, 2017
Listening and learning
Where rare disease states come with complex descriptions, the examples illustrated in this brief analysis suggest that the variety of ways in which those descriptions are expressed can be diverse. This may create a challenge for effective dissemination of knowledge and information among HCPs and between manufacturers and their customers.
Treatment manufacturers have the opportunity to learn by listening to their customers, engage in a genuine way about topics of mutual interest, and even stimulate the use of terminology that brings consistency to the conversation about emerging treatments. While international regulatory compliance obligations may hinder manufacturers from posting on public social media about products, a significant opportunity exists for manufacturers to develop an engaging open conversation about indications that could help bring clarity and even define the lexicon for diagnosis and treatment.
Data to inform this article was taken from online HCP conversations posted from July 2017 through July 2018 collected using CREATION Pinpoint®.

 By Daniel Ghinn
By Daniel Ghinn 



