Header Image Credit: ‘H&E staining in NSCLC‘ used under Attribution-ShareAlike 4.0 International
In brief:
- Non-small cell lung cancer (NSCLC) is the leading cause of cancer related deaths but has a rapidly evolving treatment landscape
- Thousands of HCPs share and discuss NSCLC trial data including ADAURA at ASCO and LungART at ESMO
- The 4 drug approvals most discussed were from Merck & Co, Novartis, Lilly and Roche
- Lung cancer screening has been a key topic for HCPs in response to the COVID-19 pandemic
- HCPs used social media to voice questions and concerns about NSCLC detection and treatment
As the leading cause of cancer-related deaths1, the treatment landscape for Non-Small Cell Lung Cancer (NSCLC) has been evolving rapidly over recent years. There was a record seven US FDA (Food and Drug Administration) approvals2 for the disease in May 2020 alone. Nevertheless, data has shown the five year survival rate for all disease stages to be 24% with over two million new cases diagnosed3 globally in 2018.
As treatment options have become more personalised due to stronger diagnostic tools, greater choices of targeted therapies and increasing knowledge of the disease, HCPs (healthcare professionals) online have been sharing their knowledge and experience. CREATION Pinpoint® identified over 80,000 HCP authored posts about NSCLC in English, from 16,000 global HCPs on Twitter.
While the NSCLC landscape discussions online spanned a wide range of topics, this article will investigate three themes that appeared throughout the year: virtual congress, product approvals and screening.
Virtual Congress
Congress meetings are always a key time for HCPs to learn from the latest trial data and with many meetings taking place virtually this year, it was no surprise to see a wealth of discourse on social media. In 2020, the top five congress meetings where HCPs discussed NSCLC were: ASCO20 (American Society of Clinical Oncology Virtual Scientific Meeting); ESMO20 (European Society for Medical Oncology Virtual Congress); AACR20 (American Association for Cancer Research Annual Meeting); BTOG20 (British Thoracic Oncology Group Annual Conference) and WCLC20 (World Conference on Lung Cancer).
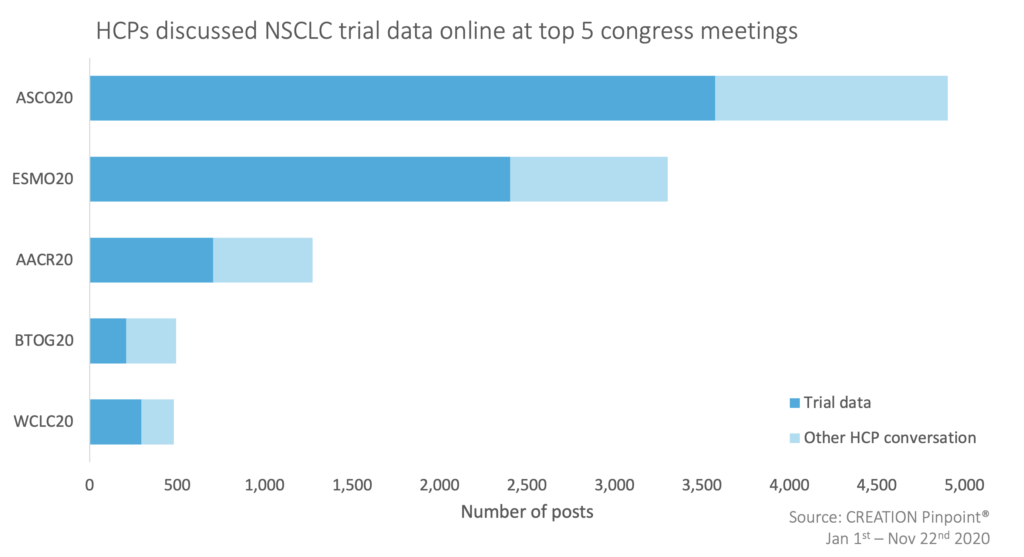
During virtual meetings, 69% of HCP conversations covered the trial data that was being shared. Among the most discussed data releases, there were 1,299 HCP mentions of AstraZeneca’s ADAURA data (616 at ASCO) for their EGFR inhibitor Tagrisso (osimertinib). HCPs celebrated the data as practice changing, including US oncologist Dr Roy Herbst, who presented the data virtually at ASCO’s annual meeting.
Presenting the ADAURA data now @ASCO @ASCOPost at the plenary session. #ASCO20 The primary endpoint was met with DFS HR=0.17! Thank you to all the coinvestigators, study team & patients for participating in this practice changing study! @AstraZeneca @YaleCancer @Yale @LungMAP pic.twitter.com/vpO2SVyLeq
— Roy Herbst (@DrRoyHerbstYale) May 31, 2020
Despite this praise, another oncologist in Italy, Dr Antonio Passaro,who shared the ADAURA data from ASCO had raised questions, including whether his peers would feel safe using the drug with their patients in mind. While still noting the high potential of the product, Dr Passaro was able to use his online platform to open dialogue around concerns he had. HCPs, in response to this question, debated the difference between DFS and PFS (disease vs progression free survival) as trial endpoints, as well as the cost benefit of the treatment.
💥 #ASCO20 #LCSM ADAURA
Clear improvement of DFS in favour OSI but many open questions are emerged.
DFS was highly significant, of course potentially practice changing but…
Would you feel safe to use Osi in adjuvant thinking about your patients/OS? pic.twitter.com/ZqKM5ZItC2— Aɴᴛᴏɴɪᴏ Pᴀssᴀʀᴏ (@APassaroMD) May 29, 2020
At ESMO’s meeting in September, a key trial discussed among HCPs was LungART, looking at postoperative radiotherapy (PORT) in NSCLC (288 mentions). According to their online posts, HCPs were surprised at the result of no significant benefit to DFS or OS (overall survival) with PORT. A tweet from US physician Dr Henning Willers provided a place for his peers to ask questions about the data, including how it would impact practice patterns.
LungART trial finally out at #ESMO20. Postop #radiotherapy with no significant benefit. Mediastinal recurrence of 46% without PORT was what would be expected & PORT decreased it but (I must say surprisingly) did not translate into DFS or OS benefit @PercyLeeMD @KHigginsMD #lcsm https://t.co/1OSSucWYVq
— Henning Willers, MD (@HenningWillers) September 20, 2020
Not only can HCPs disseminate virtual presentations from congress, but social media gives the opportunity for global HCPs to consider and discuss the practical, clinical implications of the data, as well as raise any concerns or questions they may have. Pharmaceutical companies and those conducting research can learn from their customers by listening to these online conversations and acting, in this season of digital transformation, upon the comments that arise.
Approvals
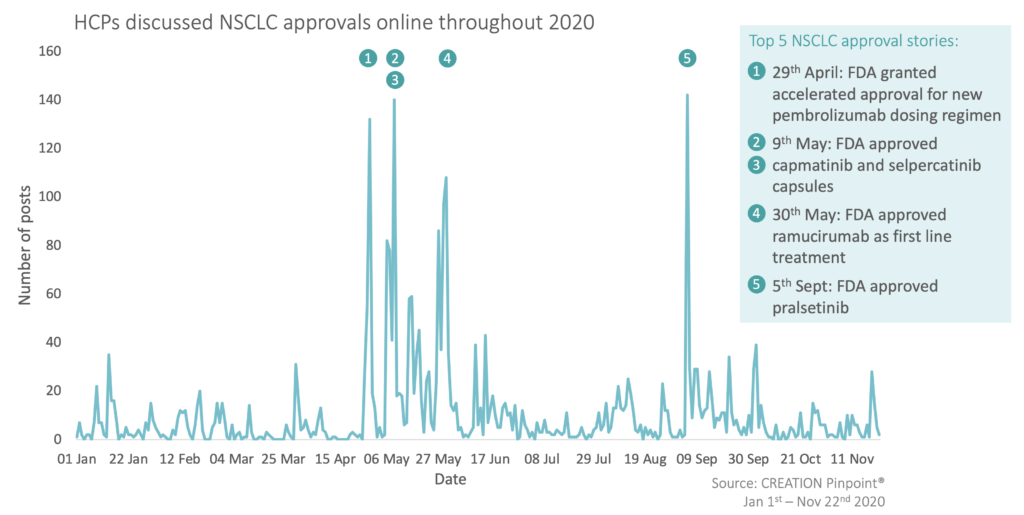
So far, 2020 has been a busy year for NSCLC approvals; May was a particularly full month with 7 new positive decisions from the US FDA. Several HCPs, including Dr Vivek Subbiah, compiled lists of the latest updates, hailing them as a “win for all patients with lung cancer”.
🥁🥁🥁By popular demand now the full list of US FDA approvals for #lungcancer from May 2020. 7 approvals ( YES!!! SEVEN) in May alone and now one for RET + NSCLC on September 4th 2020. Its a win for all patients with lung cancer. 👏👏👏 @OncoAlert #oncoalert https://t.co/FEa4gOWTmv pic.twitter.com/OjHZp1EJdF
— Vivek Subbiah, MD (@VivekSubbiah) September 5, 2020
With so many approvals, there were five that gained the most traction among HCPs (on four days). These were each FDA decisions for pembrolizumab (Merck and co), capmatinib (Novartis), selpercatinib (Lilly), ramucirumab (Lilly) and pralsetinib (Roche).
Within the celebrations of new products, HCPs began to raise issues they were facing for patient access. Thoracic oncologist, Dr Aparna Hegde, asked her peers online if they had been successful in getting Novartis’s capmatinib; she tagged the pharmaceutical company and insurance provider’s Twitter accounts to seek help, receiving responses from both.
Has anyone been successful in getting Capmatinib for their patient since FDA approval? #LCSM
— Aparna Hegde, MD (@notahedge) May 22, 2020
Product launch is a key time to listen to HCP customers online, not only do they respond to approval news and accompanying data, but they seek help and advice both directly and indirectly from the relevant companies. The weeks following key approvals are often incredibly insightful, painting pictures of the clinical perspective of HCPs who share on social media.
Screening
Over the course of this year, there have been over 4,000 HCP authored posts on Twitter advocating for lung cancer screening in order to improve disease outcomes. At the beginning of the year, UK oncologist Dr Clive Peedell shared information from BTOG20 and said that the UK was behind in their access to diagnostic tools. He suggested this has an impact on cancer survival.
Prof David Baldwin highlights the fact that UK is 3rd from bottom of the major industrialised countries, in terms of provision of CT scanners per million population. This is a key reason why UK has worse cancer survival compared to other countries. #BTOG20 #LateDiagnosis #LCSM
— Clive Peedell (@cpeedell) January 30, 2020
As the COVID-19 pandemic overtook healthcare systems globally, HCPs used their online platforms to educate others and discuss the difficulty that the pandemic had added to lung cancer detection.
Dr Viren Kaul in the US, among others, advertised a Twitter Chat (#JournalCHEST), where chest physicians would be discussing a publication on lung cancer screening during the pandemic. The journal club discussion found discrepancies across screening sites and communication errors, both factors affecting patient satisfaction.
Join us this Thursday, MAY14 at 4PM eastern for the inaugural #JournalCHEST Journal Club.
We are discussing publication by Dr. Mazzone and @LungDocDoug and team in management of lung modules and #LungCancer screening in the times of #COVID19
Link to join for free 👇🏽👇🏽 https://t.co/6mIPfz4DjM
— Viren Kaul, MD (@virenkaul) May 12, 2020
Throughout the pandemic, HCPs actively encouraged their online networks to insist on better screening for earlier detection. HCPs shared from organisations, including the UK Lung Cancer Coalition and the GO2 Foundation for Lung Cancer, who supported this messaging.
Today more than ever, we need to help, support, + elevate each other. We cannot relax on #lungcancer or any #cancer during #COVID19. We have to accommodate but insist on advancing screening, early detection, + all the progress we have seen in surgery, radiation, + medications n=1 https://t.co/7a6Rw6aX5M
— Christine Lovly, MD, PhD (@christine_lovly) October 22, 2020
Don't let COVID-19 impact our ability to diagnose #lungcancer in symptomatic patients & detect cancer by screening as shown in UK report; as we head into lung cancer action month, it's time to double down on both! @NLCRTnews @UMRogelCancer @LCAM_org @RadiologyACR @LungAssociation https://t.co/WcPeVkHrFG
— Ella Kazerooni 〽️ (@ellakaz) October 22, 2020
Pharmaceutical companies were included in these discussions when they were involved in the sharing of relevant content. AstraZeneca was mentioned by Dr John Edwards during their virtual Lung Summit reviewing lung cancer screening.
Delegates at the @AstraZeneca #ThinkAgain #LungSummit have been treated to a fabulous talk on #LungCancer #Screening by @MatthewEvison1 #LCSM pic.twitter.com/X6abpOzjoy
— John Edwards PhD FRCS(C/Th) (@_johnedwards) November 18, 2020
Historically, when HCPs include pharmaceutical companies in their online conversations, they highlight key data, whether positive or negative, as well as campaigns that resonate with existing discussions. Podcasts, infographics and DOL (digital opinion leader) video interviews have shown to be materials that successfully engage HCPs online.
In the first episode of Taking Cancer On: The Podcast, we spoke with Dr. Darryl McConnell @d_b_mcconnell about how his team of scientists are using molecular keys to unlock cancer solutions– and what this could mean for future patients. #lcsm #takingcanceron
— Boehringer Ingelheim (@Boehringer) October 23, 2020
Supporting HCP needs
2020 has been a year of advances and struggles in the NSCLC landscape in light of the COVID-19 pandemic as well as an increasing breadth of available treatments. HCPs tracked updates and challenges on their social media platforms, consulting international colleagues on key topics. Product approvals from AstraZeneca, Lilly, Roche and Novartis, among others, initiated fresh discussions on how these could impact clinical practice and standard of care, ultimately improving outcomes for NSCLC patients.
The developments and conversations for lung cancer diagnostics and treatment present a key opportunity for pharmaceutical companies to engage with their HCP customers.
CREATION Pinpoint® tracks the online HCP response to various pharmaceutical matters on a monthly basis including COVID-19 vaccine development and pharma engagement. To find out more and for bespoke HCP insights, we’d love to help.
References
1 https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html – Accessed 23/11/20
2 https://www.lungcancerresearchfoundation.org/research/why-research/treatment-advances/ – Accessed 23/11/20
3 https://www.wcrf.org/dietandcancer/cancer-trends/lung-cancer-statistics – Accessed 23/11/20