Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
The conversation Level of HCPs discussing product launches on Twitter
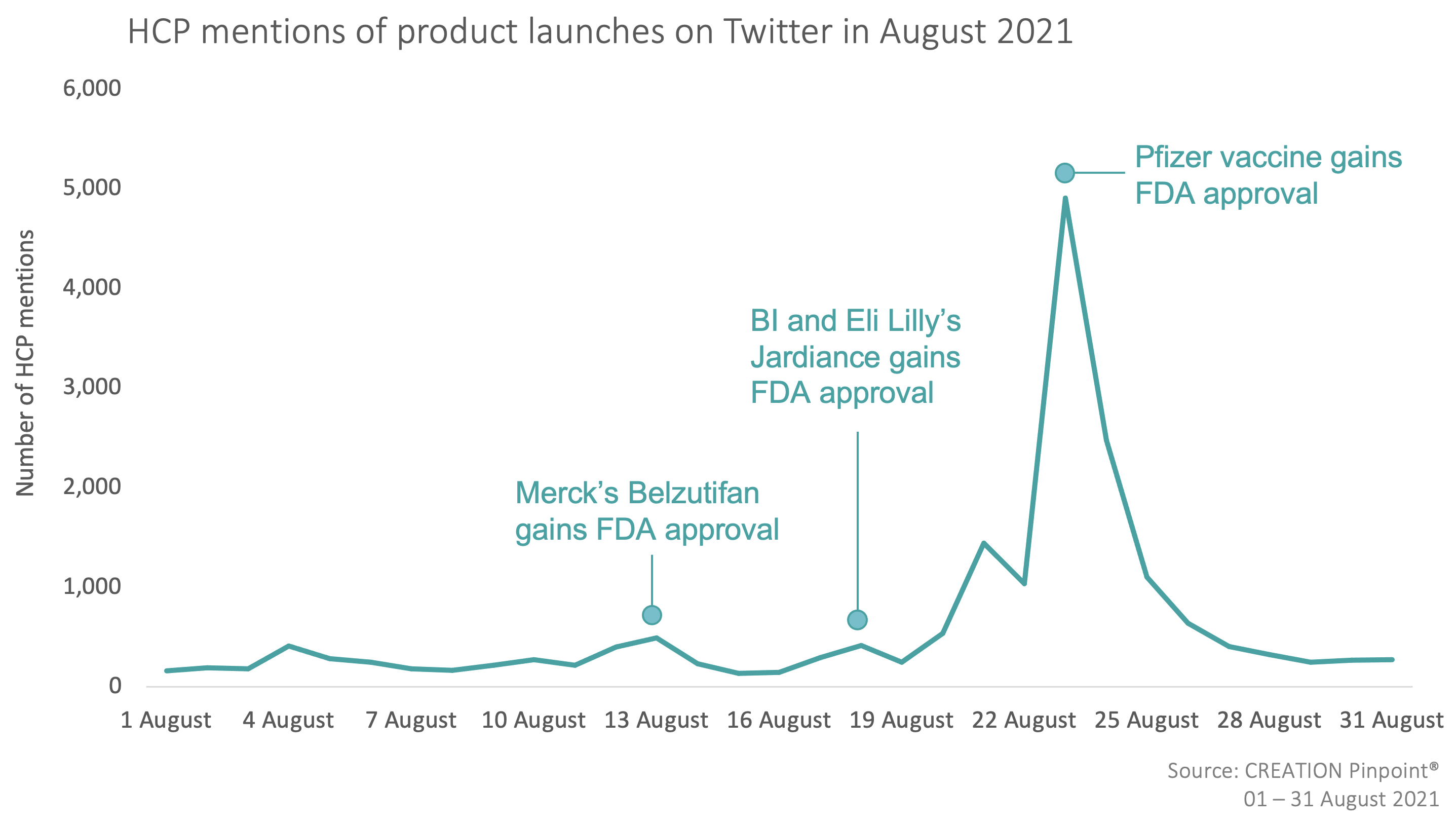
Throughout August 2021 we tracked the global conversations of 9,265 HCPs who posted 18,563 English-language Twitter posts about the launches and approvals of new products.
The latest product approval insights
In August 2021, the new product approvals that caught HCPs’ attention were Merck & Co subsidiary Peloton Therapeutics’ Welireg (belzutifan) treatment for patients with Von Hippel-Lindau syndrome (VHL), as well as Jardiance (empagliflozin), a Boehringer Ingelheim and Eli Lilly and Company product used to treat adults living with heart failure with reduced ejection fraction. However, the conversation among HCPs around these approvals was overshadowed by the discussion of the FDA’s approval of the Pfizer vaccine for the prevention of COVID-19 disease in all individuals 16 years of age or older – before this point, the vaccine had only been granted authorisation for emergency use. This caused the overall volume of HCP tweets discussing product launches in August to be more than three times higher than the previous month.
HCPs such as Emergency medicine physician, Raul Ruiz, and physician, Ashish K. Jha celebrated the news of the Pfizer vaccine gaining full approval. Many also took the opportunity to encourage people to get vaccinated. However, some HCPs felt it would still take several months for this to impact vaccine rates significantly enough to tame the virus.
I applaud our nation's medical experts and our health care heroes who have served on the frontlines of this pandemic working to stop the spread and save lives. Today's full FDA approval is a testament to their hard work and an important reminder to #GetVaccinated.
— Congressman Raul Ruiz, M.D. (@RepRaulRuizMD) August 23, 2021
This is fantastic and clearly warranted
We have SO much data on the safety and effectiveness of these remarkable vaccines
Approval will boost confidence for companies, schools, and others who want to create a safe space by requiring vaccinations
I am thrilled
Great work FDA! https://t.co/qawcsqELPQ
— Ashish K. Jha, MD, MPH (@ashishkjha) August 23, 2021
Alongside HCPs conversations on the approval of the Pfizer vaccine, many HCPs also referenced the lack of FDA approval of Ivermectin as a treatment for COVID-19 – with over 1,000 HCPs sharing the FDAs warning against the use of the drug.
#Ivermectin – If this tweet saves just one soul, it will be worth the time. Do NOT take ivermectin. It’s not effective for #COVID and it’s dangerous for humans. Get vaccinated & wear a mask. https://t.co/B4Gpwu9lmY
— John M. Talmadge, MD (@JohnMTalmadgeMD) August 22, 2021
Just your ER Doc here with a clear message:#ivermectine is NOT FDA approved nor recommended to treat or prevent COVID-19.
Vaccines, masks, and testing are methods to reduce likelihood of infection.
If infected w/ COVID-19, we have treatments. Ivermectin isn't one of them
— Elias Said, MD, FACEP (@MdFacep) August 22, 2021
Though the level of HCP engagement around the FDA approvals of Welireg (belzutifan) and Jardiance (empagliflozin) was notably lower than that around the Pfizer vaccine, those that were commenting on the news spoke positively about both drugs. Welireg was heralded as a great step forward for patients with cancers with VHL and HCPs were impressed with Jardiance’s strong trial results.
https://twitter.com/shilpasunil_rao/status/1428451302648991748
Great strides for patients with VHL-disease! FDA approves belzutifan for patients with cancers with VHL disease. Two FDA approvals in one week! @kcCURE @DrChoueiri https://t.co/HQjH2zjF1S
— Rana McKay (@DrRanaMcKay) August 13, 2021
Amazing !! A drug that was discovered @UTSWNews @utswcancer What a boon for our pts. Great drug, exceptional tolerance. @kidneycan @EJonasch @DrChoueiri FDA approves belzutifan for cancers associated with VHL https://t.co/fiZlLZyPZi
— Hans Hammers (@HHammersMD) August 13, 2021
The three most shared links from HCPs discussing product launches in June were:
- An FDA article about Why You Should Not Use Ivermectin to Treat or Prevent COVID-19.
- An FDA article about the FDA’s first approval of a COVID-19 vaccine.
- A New York Times article also about the approval of the COVID-19 vaccine
Each month, CREATION.co tracks the HCP conversation relating to new product launches. You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development, and therapy area-specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up for CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint®, the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 August and 31 August 2021 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 01 August and 31 August 2021, there were 9,265 HCP mentions of new pharmaceutical product launches and drug approvals from 18,563 unique HCP authors from around the world.
View the latest Product Launch Tracker here

