Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of online healthcare professionals (eHCPs) across the globe discussing product launches. Discover which new drug approvals eHCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout May 2023 we tracked the global conversations of 2,369 eHCPs who posted 3,402 English-language Twitter posts about the launches and approvals of new products.
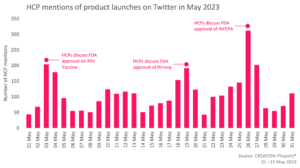
In May, several product approvals were discussed online by eHCPs. They offered their thoughts on the first oral treatment for moderate-to-severe Crohn’s disease for adults, as well as the first RSV vaccine for adults aged 60 and over.
03 May saw the FDA approve Arexvy, the first respiratory syncytial virus (RSV) vaccine approved for the prevention of lower respiratory tract disease caused by RSV in individuals 60 years of age and over. eHCPs showcased their excitement by sharing the approval, followed by positive declarations such as calling it ‘very big news’ or ‘incredible news’. One eHCP characterised this novel vaccine as a ‘long-sought scientific achievement’.
Well, this is exciting! First #RSV #vaccine #Medtwitter
FDA Approves First Respiratory Syncytial Virus (RSV) Vaccine https://t.co/lPXv4JF2QE— Thanh Neville, MD, MSHS (@thanh_neville) May 3, 2023
The FDA also approved Rinvoq (upadacitinib) for adults with moderately to severely active Crohn’s disease, just before World IBD Day. eHCPs shared positive comments around the approval news, with certain eHCPs being delighted about the first oral medication in the therapy area and others sharing their encouragement for another effective option for patients to be launched.
Rinvoq is finally here for #crohnsdisease!! https://t.co/KRbvkXGnud
— Phillip Gu (@DrPhil_Gu) May 18, 2023
Upadacitinib approved for Crohn’s disease. Another great option for our patients ! https://t.co/uE25sFm3cA
— Tauseef Ali MD, FACG AGAF FACP (@ibdtweets) May 18, 2023
Later in the month, the FDA approved Xacduro (sulbactam/durlobactam), a new treatment for hospital-acquired bacterial pneumonia, caused by strains of bacteria which are difficult to treat. eHCPs were eager to spread the news of the approval, sharing their excitement with their audience. They celebrated the approval online and congratulated their peers online for their scientific achievement.
It’s hereeeee!
FDA Approves Sulbactam-Durlobactam for Bacterial Pneumonia https://t.co/XapTTBhbf3 via @contagionlive
— Emily Heil (@emilylheil) May 23, 2023
🔥BREAKING🔥
US FDA approved Xacduro (sulbactam durlobactam for injection), a new treatment for hospital-acquired bacterial pneumonia &ventilator-associated bacterial pneumonia caused by Acinetobacter baumannii-calcoaceticus complex#IDTwitter https://t.co/HpCyNQSe1k— Antibiotic Steward Bassam Ghanem 🅱️C🆔🅿️🌟 (@ABsteward) May 23, 2023
On 19 May, the FDA granted accelerated approval to Epkinly (epcoritamab-bysp) manufactured by Genmab for treating relapsed or refractory diffuse large B-cell lymphoma (DLBCL). eHCPs were delighted about the approval and they expressed enthusiasm and stated the treatment would be a ‘wonderful addition’ to other lymphoma treatments available to patients.
Epcoritamab just approved for r/r DLBCL! Very exciting to see how this will be implemented in the community https://t.co/qy2RywdTMt
— Nikesh Shah, MD (@TheOncDoc) May 19, 2023
Well this is exciting news- will be curious on off-label use in Richter’s (or maybe it’s pseudo on label since RT is a large cell lymphoma…) EPKINLY™ (epcoritamab-bysp) Approved by U.S. Food and Drug #cllsm https://t.co/T7rLnYZXQ8
— calliecoombs (@calliecoombsmd) May 19, 2023
At the end of the month, the FDA approved Inpefa (sotagliflozin) for the treatment of heart failure. Inpefa has been granted approval for patients with heart failure or type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk factors. eHCPs were pleased to hear about the approval, sharing the ‘breaking’ news with their peers online. They commented on the promising trial data of the product, sharing their high hope for the first dual SGLT1/2 inhibitor to be approved in HF.
Breaking news: FDA has just approved sotagliflozin (brand name "Inpefa") for Treatment of Heart Failure!!!!
Broad label across full LVEF range (HFpEF&HFrEF), and for patients with or without T2DM
First dual SGLT1/2 inhibitor to be approved in HF@DLBHATTMD @ChristosArgyrop pic.twitter.com/ybWnnt4Vly
— Carlos G Santos-Gallego, MD (@SantosGallegoMD) May 26, 2023
Sotafliflozin is approved for HF! https://t.co/vpSnw3pD2V
— Christopher Cannon, M.D. 🇺🇦 (@cpcannon) May 26, 2023
The three most shared links from HCPs discussing product launches in March were:
- An FDA press release on the approval of Arexvy, the first RSV Vaccines for adults aged 60 years and over, by GSK.
- An FDA press release on the approval of Rinvoq, the first oral treatment for moderately to severely active Crohn’s disease.
- A Lexicon Pharmaceuticals announcement of the FDA approval of Inpefa, an oral tablet for treatment of heart failure.
Each month, CREATION.co tracks the eHCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest eHCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 May and 31 May 2023 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
Between 1 May and 31 May 2023, there were 3,402 eHCP mentions of new pharmaceutical product launches and drug approvals from 2,369 unique eHCP authors from around the world.
Click here to read the latest Product Launch Tracker
