Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout May 2022 we tracked the global conversations of 2,197 HCPs who posted 3,094 English-language Twitter posts about the launches and approvals of new products.
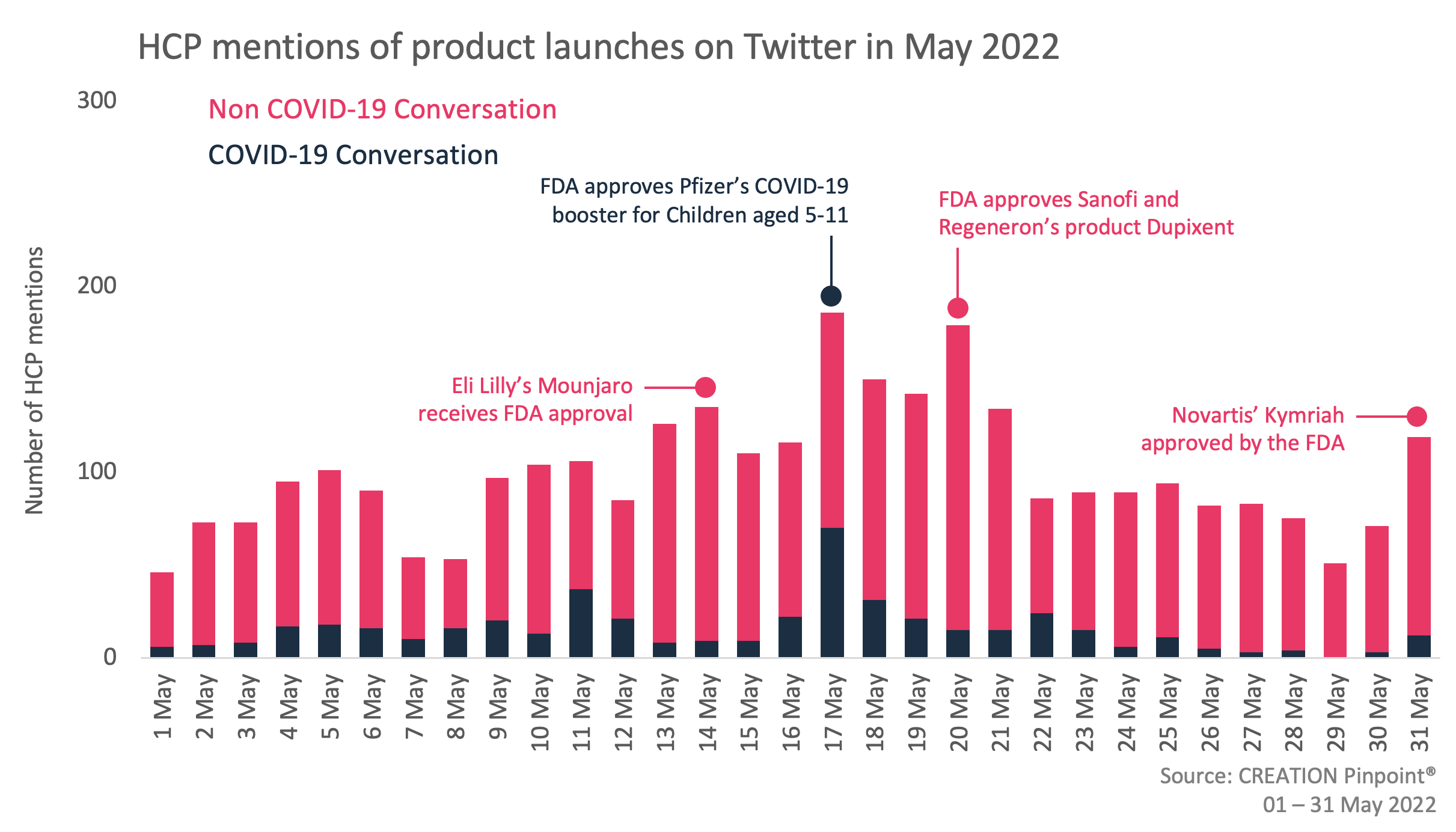
Over the last month, HCPs discussed product approvals for the treatment of a variety of diseases including lymphoma, eosinophilic esophagitis and type 2 diabetes. In addition to this, new approvals related to COVID-19 also continued to catch HCPs’ attention, with the approval of Olumiant (baricitinib) as a COVID-19 treatment and the FDA’s expansion of the full approval of Pfizer’s booster vaccine to the 5-11 age group being key talking points.
The approval that generated the most conversation among HCPs in May was Regeneron and Sanofi’s Duxipent (dupilumab), which was approved by the FDA to treat eosinophilic esophagitis (EoE), a chronic disorder of the digestive system. As the first treatment for the condition to receive FDA approval, HCPs were excited to have a new approach to managing EoE.
Dupixent is now the 1st biologic approved for treatment of Eosinophilic Esophagitis 👏
EoE is highly variable in presentation & response to treatment. There is no singular approach that helps everyone, but this now offers an additional treatment option.https://t.co/VdiZ5GDQbM
— Dr. Dave Stukus (@AllergyKidsDoc) May 21, 2022
OMG OMG OMG OMG!!!
FDA approves dupilumab as the first drug to treat eosinophilic esophagitis for adults and children ages 12 and above! #allergies #EoE #AIMedEdhttps://t.co/qHhYZbOpoI
— Zachary Rubin, MD (@rubin_allergy) May 20, 2022
HCPs also shared the approval of Eli Lilly’s Mounjaro (tirzepatide) injection, the first and only GIP and GLP-1 receptor agonist for the treatment of adults with type 2 diabetes, which is targeted to improve blood sugar control in adults. HCPs highlighted this approval as an important milestone in the obesity treatment landscape and looked forward to seeing the treatment approved for weight management in the future too, noting impressive results from the SURMONT-1 trial.
Good news, FDA Approves Novel Dual GIP/GLP1 agonist #Tirzepatide for Type 2 Diabetes. I personally much look forward it receiving FDA approval for weight management after the exciting SURMONT-1 results: 22.5% weight loss (52 lb. or 24 kg) with the 15 mg! https://t.co/CGmTkVGNmp
— Erin D. Michos, M.D. (@ErinMichos) May 13, 2022
This is big. FDA approved #tirzepatide (#Mounjaro) yesterday for diabetes, marking an important milestone in prospects for #competition in obesity treatment innovation.
HT: @LillyPadhttps://t.co/TXvV940H4k— Ted Kyle (@ConscienHealth) May 14, 2022
Alongside these approvals, developments in the COVID-19 space also continued to engage HCPs, as they discussed FDA approval of Eli Lilly’s and Incyte’s Olumiant (baricitinib) for the treatment of certain hospitalised patients with COVID-19, becoming only the second drug to have full approval for treating the virus. Intensive care doctor Wesley Ely was at the forefront of HCPs conversations about the approval, drawing attention to the positive impact the drug has already had on treating patients during the pandemic and sharing some key facts about the treatment.
Landmark FDA Drug Approval for #COVID‼️#Baricitinib: 1st Ever full approval (not Emergency Use) by @US_FDA for Life Saving “immune system medicine” in hospitalized pts on O2.
Over 12,000 pts studied since it was picked by a computer in April 2020!https://t.co/s3yW6fWP6s https://t.co/tpuLRb0ZEy
— WesElyMD (@WesElyMD) May 11, 2022
>1,000,000 have gotten Baricitinib for #COVID.
That’s ~50,000 lives saved! (pic)
POP QUIZ:💥
How many drugs have FULL @US_FDA approval for COVID?JUST TWO!
1st was remdesivir, an antiviral.
On May 10th, FDA called #Baricitinib the “first approved immunomodulator” for COVID. https://t.co/7A6SIg8EyA pic.twitter.com/zga4bXFgVD
— WesElyMD (@WesElyMD) May 12, 2022
I have read the FDAs 25 page response letter. I see why Fluvoxamine didn't get approved.
But FDA still has a problem. The bar for kids booster is WAY WAY worse than the evidence (w limits) for fluvox.
No one can possible say kids booster betterhttps://t.co/vAkhUSFyn5
— Vinay Prasad MD MPH (@VPrasadMDMPH) May 18, 2022
Finally, towards the end of May, the FDA approved Novartis’ Kymriah (tisagenlecleucel) CAR-T cell therapy for adult patients with relapsed or refractory follicular lymphoma. This made it the second CAR-T cell therapy approved for lymphoma, alongside Yescarta (ciloleucel) and the second FDA approval for this drug. HCPs generally viewed this approval as good news and a positive advancement in lymphoma treatment.
Good news: Kymriah (#Novartis) just received expedited approval by the FDA. Chimeric antigen receptors (CAR-T cells) genetically engineers the production of artificial T cell receptors in IO against cancer. Now approved for 2nd line of tx for pts w relapsed follicular lymphoma.
— Leo Nissola, MD (@LeoNissolaMD) May 31, 2022
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 May and 30 May 2022 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 May and 30 May 2022, there were 3,094 HCP mentions of new pharmaceutical product launches and drug approvals from 2,197 unique HCP authors from around the world.
View the latest and archived trackers here

 By
Paul Cranston and Tomi Shobande
By
Paul Cranston and Tomi Shobande 

