Discover what healthcare professionals (HCPs) think about pharmaceutical products and their manufacturers, as it happens, through CREATION.co’s tracking updates. How are HCPs responding to drug launch news? How are HCPs talking about or engaging with the Top 50 pharma companies on social media? Each week CREATION.co’s tracking updates bring you the latest insights from the conversation of HCPs across the globe discussing these topics and more.
June’s Top 50 Pharmaceutical Companies mentioned by HCPs on Twitter
| October Rank | Company Name | Total Mentions | Account Mentions | Account Retweets |
|---|---|---|---|---|
| 1 | Pfizer | 26,265 | 1,894 | 133 |
| 2 | Johnson & Johnson | 4,960 | 886 | 105 |
| 3 | AstraZeneca | 3,328 | 353 | 23 |
| 4 | Merck & Co | 877 | 648 | 128 |
| 5 | GSK | 773 | 354 | 43 |
| 6 | Novartis | 516 | 382 | 28 |
| 7 | Regeneron | 497 | 62 | 2 |
| 8 | Roche | 461 | 194 | 57 |
| 9 | Sanofi | 401 | 265 | 66 |
| 10 | Novo Nordisk | 332 | 300 | 13 |
| 11 | Gilead | 322 | 97 | 13 |
| 12 | Abbott | 304 | 266 | 17 |
| 13 | Fresenius | 290 | 256 | 38 |
| 14 | Bayer | 290 | 103 | 20 |
| 15 | AbbVie | 284 | 261 | 14 |
| 16 | Biogen | 261 | 129 | 4 |
| 17 | Lilly | 175 | 126 | 12 |
| 18 | Boehringer Ingelheim | 166 | 145 | 25 |
| 19 | BMS | 151 | 124 | 7 |
| 20 | Takeda | 150 | 110 | 9 |
| 21 | Otsuka | 147 | 10 | 7 |
| 22 | Amgen | 107 | 80 | 33 |
| 23 | Mylan | 98 | 4 | 0 |
| 24 | Ipsen | 75 | 67 | 5 |
| 25 | Merck KGaA | 73 | 58 | 15 |
| 26 | Teva | 70 | 54 | 21 |
| 27 | Vertex | 69 | 62 | 0 |
| 28 | Allergan | 63 | 8 | 0 |
| 29 | UCB | 52 | 46 | 25 |
| 30 | Bausch | 51 | 16 | 0 |
| 31 | Daiichi Sankyo | 47 | 33 | 7 |
| 32 | CSL | 38 | 37 | 3 |
| 33 | Servier | 38 | 7 | 1 |
| 34 | Menarini | 31 | 14 | 6 |
| 35 | Astellas | 29 | 20 | 4 |
| 36 | Chugai | 29 | 5 | 5 |
| 37 | Eisai | 17 | 4 | 0 |
| 38 | Sun | 10 | 4 | 0 |
| 39 | Alexion | 7 | 5 | 0 |
| 40 | Grifols | 7 | 0 | 0 |
| 41 | Aurobindo | 3 | 0 | 0 |
| 42 | Sumitomo Dainippon | 3 | 0 | 0 |
| 50 | Yunnan Baiyao | 0 | 0 | 0 |
| 50 | Mitsubishi Tanabe Pharma | 0 | 0 | 0 |
| 50 | Jiangsu Hengrui | 0 | 0 | 0 |
| 50 | Ono | 0 | 0 | 0 |
| 50 | Endo | 0 | 0 | 0 |
| 50 | Meiji | 0 | 0 | 0 |
| 50 | Sino | 0 | 0 | 0 |
| 50 | Shanghai | 0 | 0 | 0 |
June’s insights from HCPs mentioning the Top 50 Pharmaceutical Companies
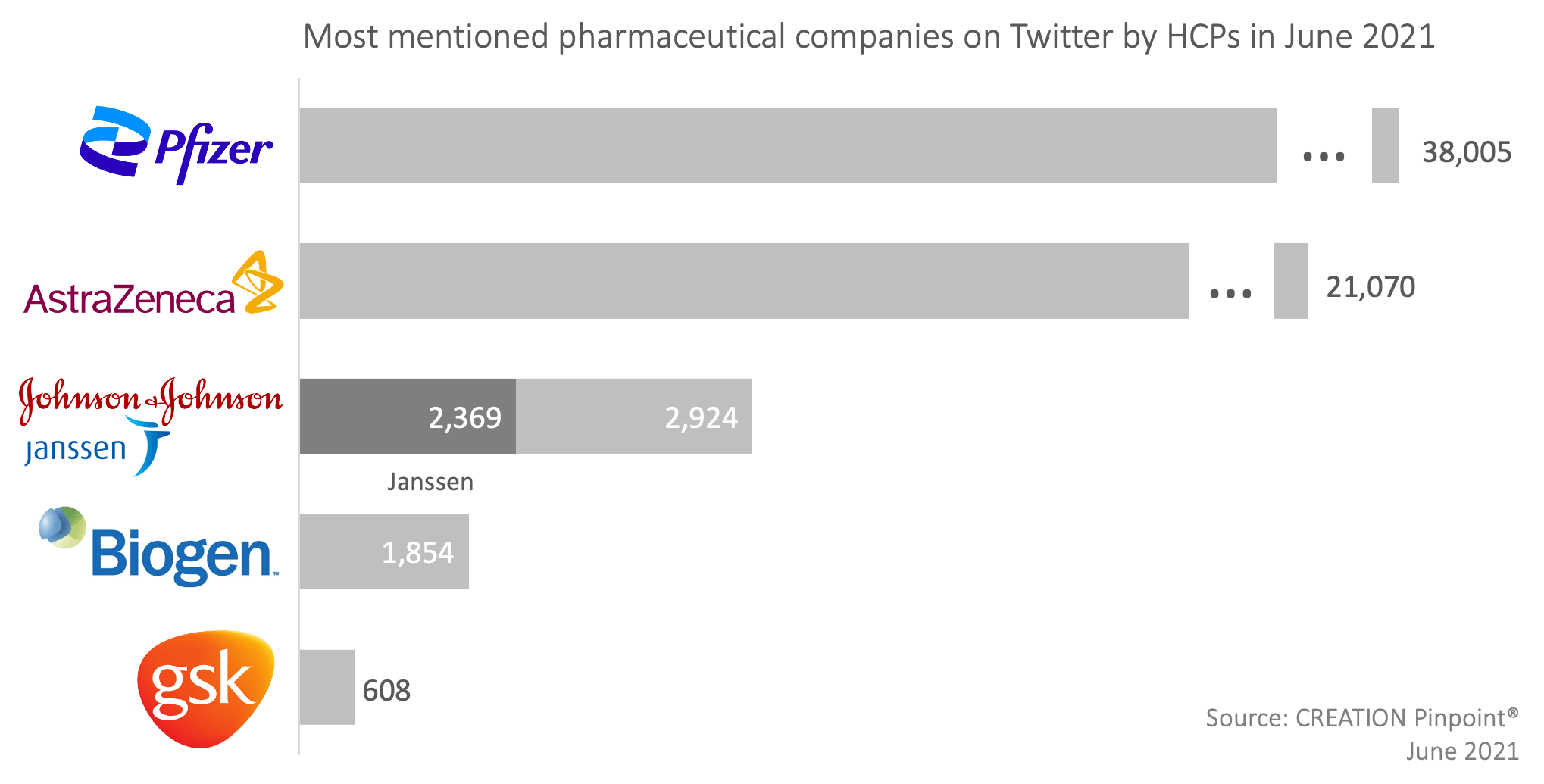
In the month of June, the top three pharmaceutical companies mentioned by healthcare professionals (HCPs) online remained the same as last month, while Merck & Co was displaced by Biogen and GSK moved to fifth place. This is the first time during this year that Biogen made it to the top five mentioned companies. The final ranking was Pfizer, AstraZeneca, Johnson & Johnson (J&J), Biogen, and GSK.
The HCP conversation this month decreased by 15% compared to the month of May, making it the second consecutive month this year in which there is a decrease in mentions of pharmaceutical companies. Nonetheless, HCPs continued to discuss the efficacy and safety of COVID-19 vaccines. This time they focused on the effectiveness that vaccines have against the Delta variant as well as the immunity response that people develop from receiving first and second doses of different vaccines.
As the Delta variant continues to be a threat to the global health response against COVID-19, many HCPs discussed the effectiveness of the Pfizer and AstraZeneca vaccines against this variant. They shared real world data from Public Health England indicating the Pfizer vaccine is 94% and 96% effective after the first and second dose of the vaccine, and AstraZeneca is 71% and 92% effective against this variant.
Here’s the key table from @PHE_uk new report on vaccine efficacy against hospitalisation with Delta variant:
After 1 dose
• Pfizer: 94% effective
• AstraZeneca: 71% effectiveAfter 2 doses
• Pfizer: 96%
• AstraZeneca: 92%Full preprint here: https://t.co/JQQjo0gSdg pic.twitter.com/osSjIZ0eA6
— John Burn-Murdoch (@jburnmurdoch) June 14, 2021
HCPs were also active in discussing various governments’ approaches to mix the first and second dose of different vaccines. They shared data showing that giving individuals a first dose of AstraZeneca and a second dose of Pfizer results in a robust immunity response against COVID-19. They also mentioned that this approach still needs approval from the FDA.
UPDATED #MIXANDMATCH #COVID19 VAX FINDINGS: AZ & PFIZER. New data from the ongoing CombiVacS study: adults aged 18-60 with a first dose of #AstraZeneca were given a second dose using #Pfizer after 8-12wks. Result: safe & effective. (❗Needs FDA approval)https://t.co/FsPa8FipDR pic.twitter.com/bjYGMQHqKx
— Albert Domingo (@AlbertDomingo) June 25, 2021
Just published @TheLancet
Largest mix-and-match, randomized, clinical vaccine trial of people with AZ first dose, Pfizer 2nd dose. Safe and notable improved neutralizing antibody and T cell response with mix vs controls (no 2nd dose) https://t.co/L7XjnJXiDC pic.twitter.com/66MDN21j3R— Eric Topol (@EricTopol) June 25, 2021
The three most shared links by HCPs in June were:
- An article published by Public Health England (PHE) showing the effectiveness of COVID-19 vaccines against hospital admission with the Delta variant.
- A press release from the UK government showing the PHE analysis of the effectiveness against the Delta variant after two doses of the COVID-19 vaccine.
- An article of a phase two trial published in the Lancet showing the results of combining the Pfizer and AstraZeneca vaccine against COVID-19.
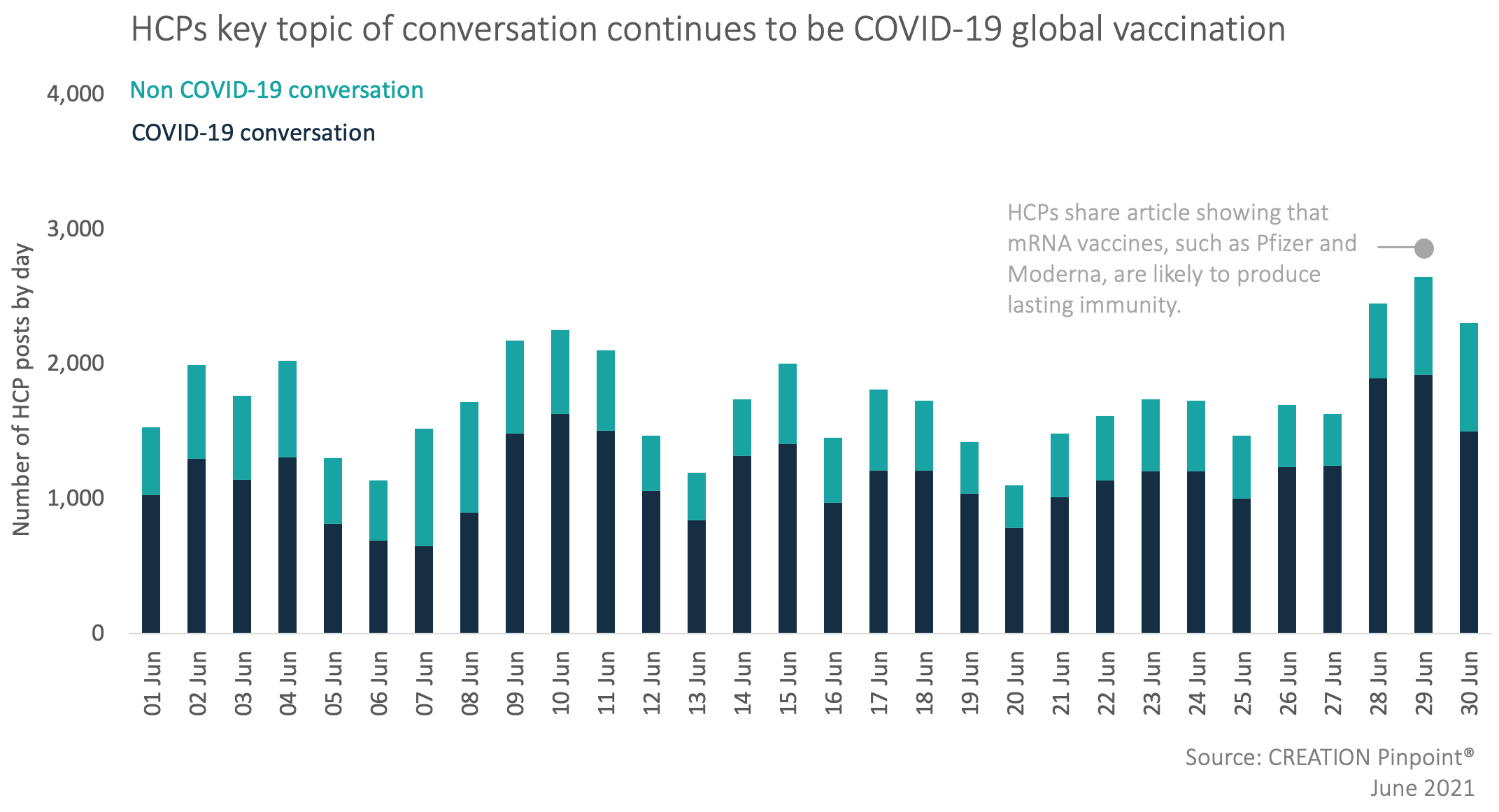
Between May and June the volume of HCP COVID-19 conversation in the context of top 50 pharma decreased by over 7,500 posts, making up 69% of the overall HCP conversation in June.
Outside of the COVID-19 conversation, HCPs shared Novartis’ VISION trial results, which were said to be a “huge milestone” for radiopharmaceutical theranostics. The results showed impressive survival results of 177Lu-PSMA-617 for late-stage prostate cancer patients. HCPs also shared the new FDA approval for the drug Aduhelm (aducanumab) manufactured by the pharmaceutical company Biogen.
This approval came with negative responses by HCPs as they said the trials from which the FDA decision had “not enough evidence to support the approval”. This resulted in HCPs asking for another trial to be done in order to make sure patients are safe. Additionally, HCPs showed resistance about the price of this drug, which is known to cost $56,000 per year per patient.
Novartis VISION trial results are out.
Huge milestone. This will pave the way for a whole new era of radiopharmaceutical theranostics.
Many more applications, indications, refinements and new targets to come.https://t.co/uiYMjEfJFS pic.twitter.com/taVEFHNCze— Jeremie Calais (@CalaisJeremie) June 3, 2021
The FDA approved an Alzheimers drug made by Biogen even though trials showed no benefit. The cost for this IV drug will be $56,000 per year per pt plus the cost of diagnostic tests of about $30,000 the 1st year and $15,000 each year after. Who exactly is the FDA working for?
— Henry M. Rosenberg (@DoctorHenryCT) June 8, 2021
10 out of 11 people on an FDA advisory panel found "not enough evidence" to support approval of Biogen's Alzheimer's drug. The 11th panellist was “uncertain.” Yet, the @US_FDA overruled the advice & approved the drug anyway, sparking a resignation.https://t.co/5Vj3xBXa9M
— Maryanne Demasi, PhD (@MaryanneDemasi) June 9, 2021
CREATION.co continues to analyse online HCP conversation on a variety of topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
- In May 2021, CREATION Pinpoint® identified 52,204 healthcare professional (HCP) authored tweets from 16,524 individual HCPs mentioning a Top 50 pharmaceutical company (according to revenue).
- Data for this research was analysed from the online Twitter conversations of HCPs in all languages mentioning a Top 50 pharmaceutical company between June 1st and June 31st 2021.
- Unless otherwise specified, mentions of a company are not limited to its Twitter account(s).
- In some cases, a tweet may mention more than one company account (eg. @abbottnews and @abbottglobal) – this only counts as one Company Mention and Account Mention.
- Johnson & Johnson was included as a whole, encompassing its pharmaceutical subsidiary, Janssen. In June, 2,369 of these posts mentioned Janssen.
 By Mary Kangley
By Mary Kangley