Every month, CREATION.co’s tracking updates bring you the latest insights from the online conversation of healthcare professionals (eHCPs) across the globe discussing product launches. Discover which new drug approvals eHCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout November 2023 CREATION.co tracked the global conversations of 1,633 eHCPs who posted 2,313 English-language X (formerly Twitter) posts about the launches and approvals of new products.
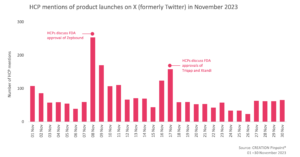
In November, 166 more eHCPs discussed treatment approvals compared to October and there were 14% more eHCP mentions of new pharmaceutical product launches and drug approvals.
On 8 November, the FDA approved Eli Lilly’s Zepbound (tirzepatide), for chronic weight management for adults with obesity. Tirzepatide, the active ingredient in Zepbound, is already approved under the trade name Mounjaro, for type 2 diabetes. eHCPs were delighted by the news of the approval, as they felt that Zepbound would be a powerful new option offering patients effective treatment. A few eHCPs also commented on Zepbound being priced 20% lower than Wegovy, saying they hope it will lead to better access and sustained lower pricing.
FDA approved “Zepbound”for weight loss, also known as Mounjaro for Diabetes. This medication has been a game changer for many of my patients. It reduces one’s risk for diabetes, heart disease, fatty liver disease, cancer and many other health conditions. I’ve even had patients… pic.twitter.com/xsBrLDiezT
— Dr. Becky Chandler (@DrBecC) November 8, 2023
Eli Lilly's new weight loss drug Zepbound, originally Mounjaro for diabetes, gets FDA nod. It's set to rival Novo Nordisk's Wegovy, offering up to 21% weight loss in trials, at a lower price. @EliLillyandCo @elaineywchen @damiangarde https://t.co/6SeUSizUNK via @statnews
— Ashkan Afshin (@aafshinmd) November 8, 2023
On 16 November, the FDA approved Pfizer and Astellas’ Xtandi (enzalutamide) for non-metastatic castration-sensitive prostate cancer (nmCRPC). Two months prior, on 12 September, the EMA had also approved Xtandi for the same indication. eHCPs showed their excitement around the news on X and also shared the OncoAlert newsletter which was discussing Xtandi’s FDA approval. Dr Chandler Park called the treatment “practice changing” while many other eHCPs congratulated their peers on the EMBARK clinical trial, which was assessing the treatment.
🔬 Exciting news in prostate cancer treatment! The FDA just approved enzalutamide for non-metastatic castration-sensitive prostate cancer with biochemical recurrence. The EMBARK trial showcased its impact on delaying metastasis, marking a significant leap in treatment options.…
— Emad Shash (@emadshash) November 17, 2023
Breaking news👉 @US_FDA approves enzalutamide with/without androgen deprivation therapy (ADT) for non-metastatic castration sensitive #ProstateCancer based on Embark trial data👇Congrats @nealshore @SFreedlandMD Link👉 https://t.co/fIdluT0NLv @PCFnews @OncoAlert @urotoday pic.twitter.com/YNKYEYjyxS
— Neeraj Agarwal, MD, FASCO (@neerajaiims) November 17, 2023
Also on 16 November, the FDA announced the approval of AstraZeneca’s Triqap (capivasertib with fulvestrant) for the treatment of HR+/HER2- metastatic breast cancer. As it was approved, Dr Paolo Tarantino sparked a conversation about a sub-analysis of the CAPItello-291 trial which will be presented at SABCS23. Overall, eHCPs were supportive of the approval as it brings another treatment option for breast cancer patients.
Big news: approval in the altered population only. Great to have this new option! @OncoAlert FDA approves capivasertib with fulvestrant for breast cancer | FDA https://t.co/J6msOP5x84
— Hope Rugo (@hoperugo) November 16, 2023
🚨FDA approves capivasertib with fulvestrant for adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN-alterations
👉CAPIVASERTIB name is TRUQAP…
— Vivek Subbiah, MD (@VivekSubbiah) November 17, 2023
The three most shared stories from eHCPs discussing product launches in November were:
- An Eli Lilly press release on the approval of Zepbound for the treatment of obesity and weight management.
- A CISION PR Newswire news article on the approval of Xtandi for the treatment of prostate cancer.
- An FDA press release on the approval of Triqap for the treatment of HR+/HER2- metastatic breast cancer.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language X conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 November and 30 November 2023 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their X conversations.
- Between 1 November and 30 November 2023, there were 2,313 eHCP mentions of new pharmaceutical product launches and drug approvals from 1,633 unique eHCP authors from around the world.
Top 50: https://creation.co/resources/hcp-insight-trackers/top-50-pharma-tracker/
Product approval: https://creation.co/resources/hcp-insight-trackers/product-launch-tracker/
All trackers: https://creation.co/resources/hcp-insight-trackers/
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou 

