Each month, CREATION.co’s COVID-19 vaccine tracker brings you the latest insights into the online healthcare professionals (HCPs) responding to the latest news and trial results for COVID-19 vaccine candidates.
Discover what healthcare professionals (HCPs) think about pharmaceutical products and their manufacturers, as it happens, through CREATION.co’s tracking updates. Each week CREATION.co’s tracking updates bring you the latest insights from the conversation of HCPs across the globe discussing Top 50 pharma, respiratory disease and more.
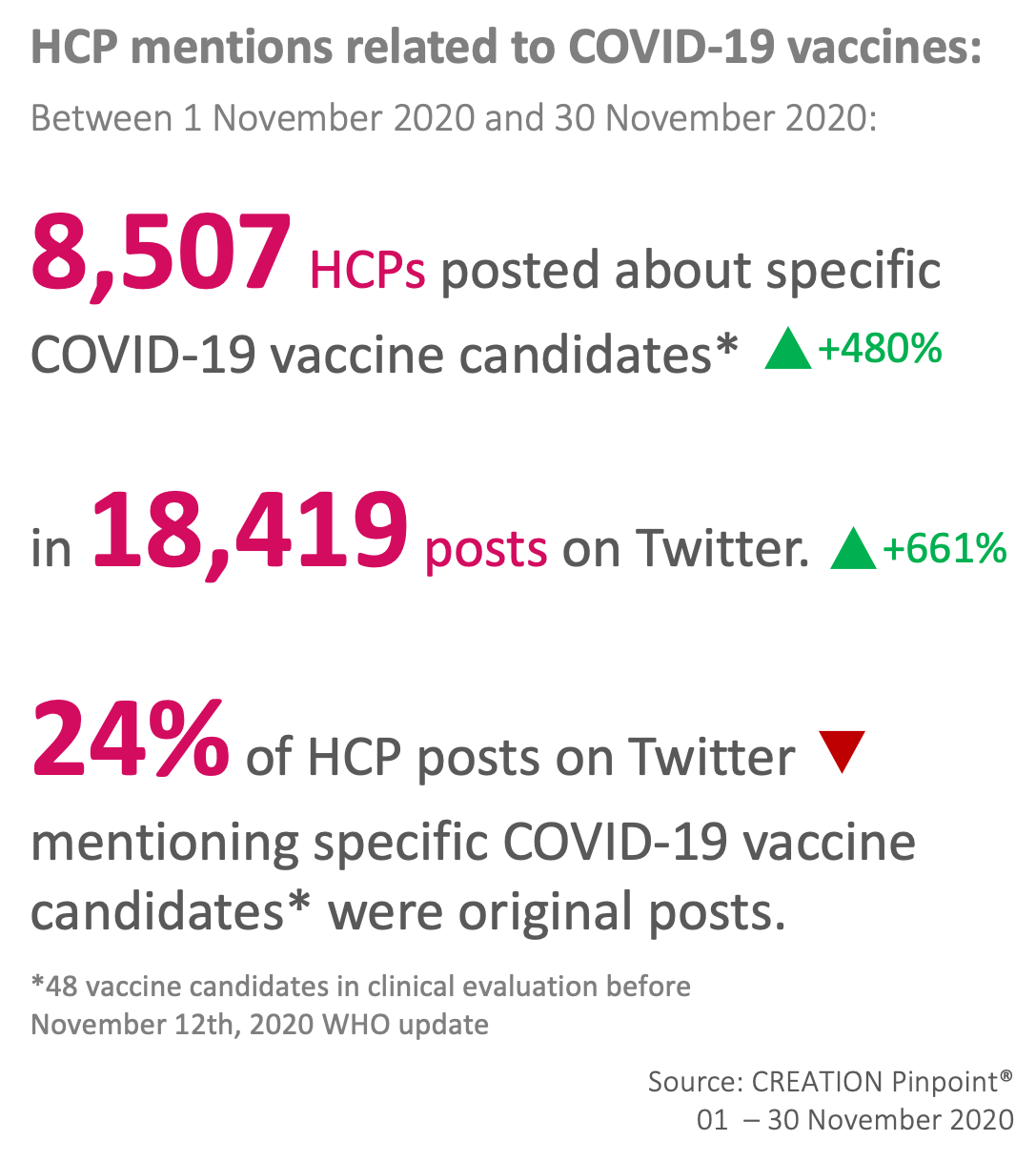
The Top 10 covid-19 vaccine candidates mentioned by HCPs on Twitter in November
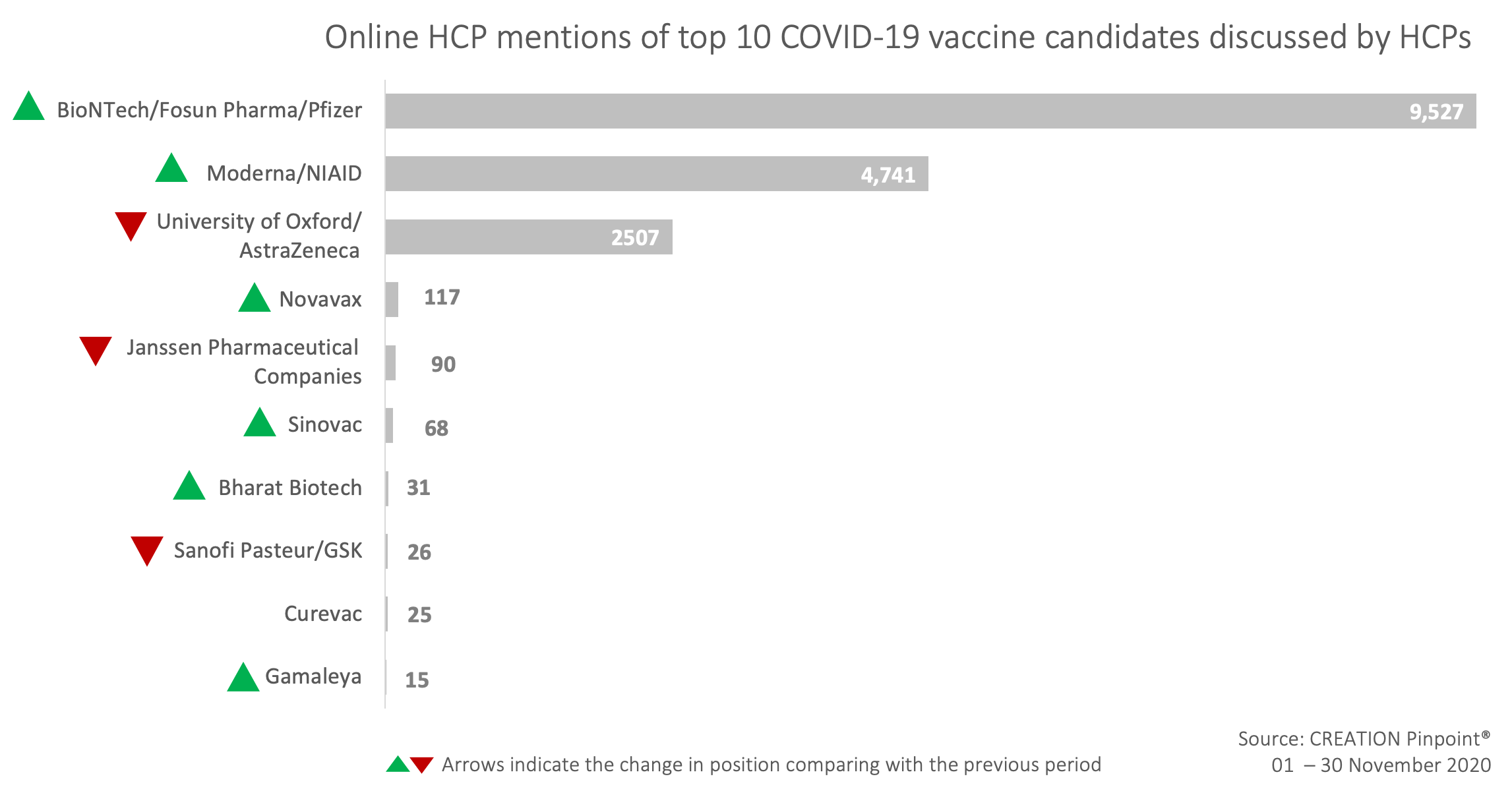
November’s insights from HCPs mentioning covid-19 vaccine candidates on Twitter
November has seen a huge increase in volume of conversation around COVID-19 vaccine candidates, driven primarily by news of vaccine efficacy shared by developers who have consolidated their final datasets from clinical trials. Whilst Pfizer overwhelmingly took up the lion’s share of HCP discussion, much of the conversation has concerned itself with weighing the pros and cons of each candidate, the novelty of the mRNA treatment, and what challenges now face HCPs and patients from a public health perspective.
Prominent HCPs posted explanations covering the mechanisms of Pfizer’s new vaccine, which uses RNA sequencing to develop our body’s immune response to the virus.
The most retweeted post about a vaccine candidate by HCPs in November was from Rand Paul, a US politician who offered information about Pfizer and Moderna’s vaccine trial results and urged we support efforts to administer the vaccine.
So far, evidence indicates only a handful out of 11 million COVID patients may hav been reinfected. That’s actually lower than rate of infection with Pfizer’s new vaccine! CoVID patients are overwhelmingly immune, and science deniers should stop pretending otherwise.
— Rand Paul (@RandPaul) November 14, 2020
In addition to politicians commenting on issues in the healthcare sector, last month’s COVID-19 vaccine candidate news also prompted many HCPs to comment on political factors that bear on the deliverance of a successful vaccine. Cost is undoubtedly an important factor for many, though others have focused on the need for a robust public health strategy and a level of trust between scientists and public officials.
How are scientists and public health officials building trust in a vaccine for COVID-19? @angie_rasmussen and @jevinwest join us. https://t.co/dvd6cDwJxU
— 1A (@1a) November 30, 2020
AstraZeneca maintains appeal despite inferior efficacy
AstraZeneca did not dominate the vaccine candidate conversation last month, in part due to news of the results from their collaboration with Oxford arriving later in the month. Despite results that on the face of it appear to be less impressive than Pfizer and Moderna (70% efficacy vs. 95% efficacy), closer analysis reveals a number of favourable factors that HCPs have focused on.
Firstly, HCPs have noted that it is much cheaper and easier to store, two barriers of access to states wishing to purchase the vaccine.
Secondly, one dosing regimen demonstrated 90% efficacy in the interim results, suggesting that AstraZeneca may not be as far behind in efficacy than might first be assumed.
📍Two key takeaways why the Oxford #COVID19 vaccine could be better than Pfizer/Moderna:
📌Refrigerator storage (no freezing needed, unlike mRNA ones by Pfizer & Moderna)
📌Just $2.50 per dose for Oxford/AZ vaccine ($15-25 a dose for Pfizer & Moderna mRNA vaccines).
🧵summary: https://t.co/2aZHvlUBCK pic.twitter.com/1yclsXRJsm
— Eric Feigl-Ding (@DrEricDing) November 23, 2020
Looks like the AstraZeneca #COVID19 vaccine may work too.
Interim results demonstrated that one of the dosing regimens had 90% efficacy.
No hospitalizations or severe cases in those who received vaccine.
Incredible news, and now 3 hopeful vaccines.https://t.co/mx8T2vumDA pic.twitter.com/vJaAbcp2sc
— Isaac Bogoch (@BogochIsaac) November 23, 2020
As the first COVID-19 vaccine candidates near completion, and the issue at hand turns from developing a successful vaccine to administering a successful vaccine, logistical problems will continue to be a concern for HCPs, as well as ensuring positive support from governments and media with regards to the vaccination programme.
With vaccine development efforts in full steam, and distribution on the horizon, there are still many obstacles in the way of a successful vaccine administration. HCPs have played a significant role in public communication during the pandemic so far, and many have actively indicated a need for more trust and partnership between HCPs and pharma. Insofar as their online behaviours are reflections of their wants and needs, understanding HCP digital communication continues to be invaluable for pharmaceutical companies at this time.
We will be tracking the online HCP conversation to identify trends and change in views relating to COVID-19 vaccines. You can stay up to date with HCP insights by subscribing to CREATION Knowledge e-journal.
- Data for this research was analysed using CREATION Pinpoint® from the online Twitter conversations of HCPs around the world in English language (other languages are available), between November 1st – November 30th, 2020.
- Vaccines tracked were the 48 COVID-19 vaccine candidates in clinical evaluation before 12th November 2020 (WHO Draft landscape of COVID-19 candidate vaccines).
