The annual American Society of Clinical Oncology (ASCO) conference, held in Chicago this year, gained over 40,000 healthcare professional (HCP) registrations – both in person and online. Online HCPs (eHCPs) frequently take to social media to discuss data released at large global events like ASCO, so we were curious to find out what eHCPs were discussing in the lung cancer conversation over the month before and during the ASCO conference 2023.
Using CREATION Pinpoint® we analysed the unprompted lung cancer conversations of 2,632 eHCPs between 1st May to 8th June 2023 globally. Over 9,000 social media posts were made discussing lung cancer over this time period, 43% of which fall into the time frame of ASCO – 2nd to the 6th of June. 37% of the lung cancer conversation over this period spoke specifically about non-small cell lung cancer (NSCLC) , with only 4% of the conversation about small cell lung cancer (SLC).
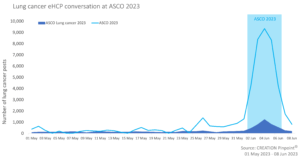
The lung cancer conversation made up 15% of the total ASCO 2023 conversation online between 1st May 2023 and 8th June 2023.
ADAURA trial the most discussed by eHCPs at ASCO 2023
Over one third of the eHCP lung cancer conversation discussed research and data. The most discussed clinical trial was the ADAURA trial, investigating adjuvant osimertinib versus placebo in EGFR-mutated stage IB-IIIA NSCLC. eHCPs had mixed sentiment towards the data with some eHCPs, such as US oncologist Eric Singhi, commending the team for “offering a plain language summary”. On the other hand, some eHCPs shared their disappointment for not utilising a standard of care control arm, with one oncologist, Dr Amol Akhade, stating that it was painful to see the explanation given on podium at ASCO 2023.
👏🏽 I want to commend the #ADAURA research team for offering a plain language summary in @NEJM
‼️ Patient education matters @OncoAlert @OncBrothers @LungCancerRx #ASCO23 #lcsm https://t.co/Fckp6SUYIW pic.twitter.com/Ft4Tv2IAxq
— Eric K. Singhi, MD (@esinghimd) June 8, 2023
1 – Most of the oncologist know that most of the control arm of ADAURA did not get Osimertinib on progression ( less than 40 %) and there is no reasonable explanation for the same .
( it was painful to see the explanation given on podium in #asco23 )
2 – Number of oncologist… pic.twitter.com/5Tk3edB8Gn— Dr Amol Akhade (@SuyogCancer) June 7, 2023
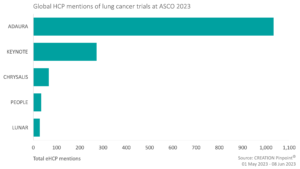
Another highly discussed clinical trial among eHCPs over the period of ASCO was the KEYNOTE trial, investigating Merck’s anti-PD-1 therapy KEYTRUDA in patients with TKI-resistant, EGFR-mutated metastatic, nonsquamous NSCLC. Generally, eHCPs appeared underwhelmed at both the results and decisions on the logistics such as the pathologic criteria of trial participants.
Although the other top five most discussed trials over ASCO 2023 had significantly fewer mentions than ADAURA and KEYNOTE, there was a general sentiment of enthusiasm towards the CHRYSALIS, PEOPLE, and LUNAR trials. eHCPs were keen to see more data on these newly reported trials.
#ASCO23 @ASCO
👇🏽Today at Opening #LCSM CSS #CHRYSALISImportant Question: METIHC or FISH in Progression on Osimertinib in #EGFRMT #NSCLC @BenjaminBesseMD @Jbauml @OncoAlert @HemOncToday @LungCancerRx https://t.co/QFOEAQfENG
— Charu Aggarwal, MD, MPH, FASCO (@CharuAggarwalMD) June 2, 2023
AstraZeneca and MSD were the focus of eHCPs during ASCO
Mentions of AstraZeneca and MSD in the context of NSCLC were driven primarily by their clinical trials.
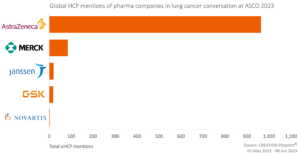
When they weren’t discussing clinical trials, eHCPs shared updates on activities occurring within the top pharmaceutical organisations, such as webinars sponsored by AstraZeneca and news updates.
However, 98% of the conversation on the top five pharmaceutical organisations discussed research and data covered at ASCO.
⭐️Press release update from #FLAURA2 trial for EGFR mutant NSCLC👉🏼Positive high-level results from the FLAURA2 Phase III trial showed AstraZeneca’s Tagrisso (osimertinib) + chemotherapy demonstrated a statistically significant & clinically meaningful improvement in… pic.twitter.com/SUPyQaS7ua
— Vivek Subbiah, MD (@VivekSubbiah) May 17, 2023
Both amplifiers and creators are among the top 5 HCP voices at ASCO 2023
Among the top voices in the lung cancer conversation at the event there were both amplifiers, who shared others’ posts the majority of the time, and creators, who created original content.
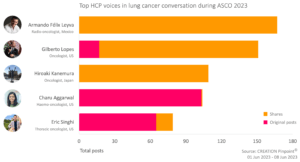
The author who contributed the most social media posts in the ASCO 2023 lung cancer conversation was Armando Félix Leyva, a radio-oncologist from Mexico. The majority of his posts were shares of peer content. 22% of his posts were about the ADAURA and KEYNOTE clinical trials, sharing research and data presented at ASCO, whilst the rest of his posts shared presentations by fellow HCPs at ASCO without mentioning specific trials.
Interesting presentation🥳 by Rebecca Suk Heist, MD confirming and reviving the standard of non-small cell #lungcancer. #ASCO23 @LungCancer_Can @AEACaP @FrentePulmonMx pic.twitter.com/gaUbqdH0DF
— Dr. Iván R. González (@Dr_Ivanoncologo) June 6, 2023
A single-arm, phase I study of sotorasib plus carboplatin-pemetrexed
in advanced non-squamous, non-small cell lung cancer patients with KRAS G12C mutation: ‼️ORR 89% ‼️WJOG14821L #ASCO23 #LCSM pic.twitter.com/p4vn2DWtIw— Dr. Antonio Calles 🫁🚭 (@Tony_Calles) June 6, 2023
Charu Aggarwal from the US, was the top creator in the ASCO lung cancer conversation, with 99% of her posts being original content. She was also interested in research and data presented at ASCO, keeping her network up to date on the details.
The top 5 posts focused on trial data
The top most impactful lung cancer posts over the period of ASCO, based on their global reach, were updates on the clinical trial data. These posts reached the screens of over 6 million social media users.
The top 5 posts are:
- Eric Topol – ADAURA trial
- Eric Topol – ADAURA trial
- Vinay Prasad – ADAURA trial
- Eric Topol – lung cancer screening
- Darren Markland – causes of cancer
Eric Topol from the US shared 3 of the top 5 posts, sharing his enthusiasm about trial results with his almost 700,000 followers on Twitter, each including a visual such as an article link or an image of the data released.
A mutation in the EGFR gene is found in ~25% of lung cancers. Reported @NEJM and @ASCO today: a pill directed at this mutation cut deaths by 50% through 5 years in a randomized trial following surgical resectionhttps://t.co/XqjSiIWTfd pic.twitter.com/JxtCaFGrMP
— Eric Topol (@EricTopol) June 4, 2023
What could your next steps be following ASCO 2023?
Firstly, understand what your HCPs are thinking about your data. Don’t shy away from negative comments, as those are good insights too and could even be a conversation starter if you want to get in touch with individual HCPs.
Secondly, taking the ADAURA trial as an example, HCPs liked the plain language summary but were questioning the control arm and were not happy about the explanation on the podium. AstraZeneca has the opportunity to address some of those concerns raised by HCPs. Similar to an “Ask the Expert” session, we have seen pharmaceutical companies hosting TweetChats on specific clinical trial data to engage the HCP community and give them an opportunity to ask questions and get answers.
Finally, make sure to track those eHCP conversations on an ongoing basis, not only over congress and when important trial updates happen. If you start from when you announce a new drug candidate, understanding the HCP voice can inform your brand vision and strategy.
If you would like to learn more about understanding, engaging and tracking the views and needs of HCPs online then please do get in touch, we’d love to chat.
 By Hannah Ghinn
By Hannah Ghinn 


