A key area of concern in oncology is diagnosing cancer and finding it early enough when it is at its most curable, something we’ve seen in our own research tracking healthcare professionals’ (HCPs’) conversations and in supporting our pharmaceutical clients. Last year, in 2019, we researched the HCP conversations in the pancreatic cancer space. In a disease that is notoriously known for late diagnosis and poor survival, it is no surprise that a large proportion of the online conversation by HCPs was about earlier diagnosis. Not only were HCPs stressing the importance of earlier diagnosis, they were also engaging their peers cross functionally on the correct tests for earlier diagnosis, with liquid biopsy being a core to the early diagnosis conversation.
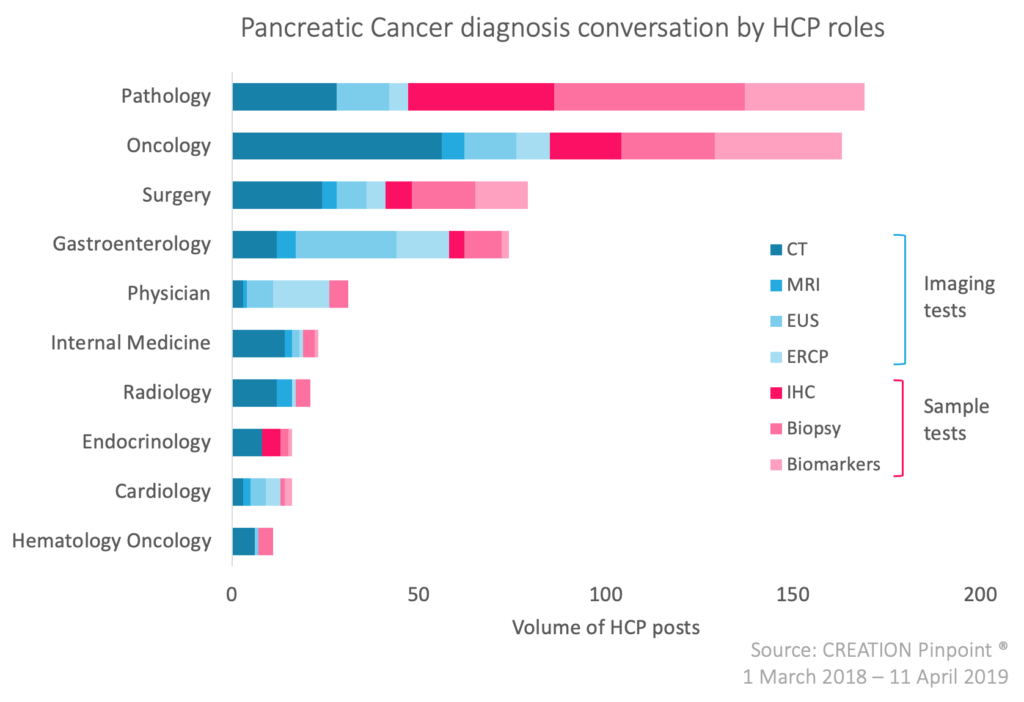
Within liquid biopsy, circulating tumour DNA (ctDNA) has been discussed by HCPs a great deal more recently. Over the months of August to September 2020, and during the medical congress held by the European Society for Medical Oncology (ESMO) in late September, a number of studies were presented sharing results of the use of ctDNA. This time the conversation had a different focus, with HCPs discussing the prognostic capabilities of patient response to cancer treatments using ctDNA.
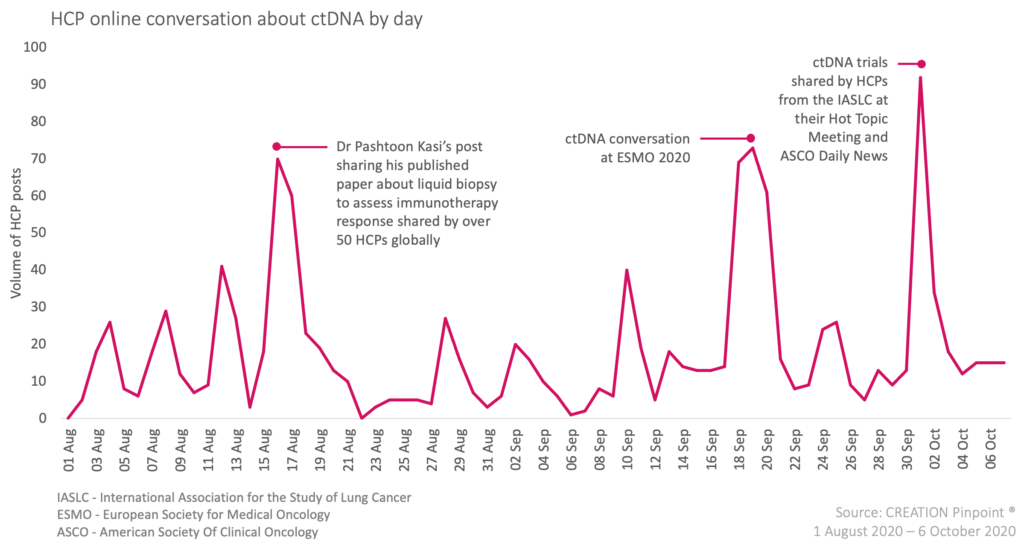
ctDNA testing revolutionary to cancer care
Led by GI oncologist, Dr Pashtoon Kasi, many HCPs shared that liquid biopsies and in particular ctDNA are revolutionising cancer care.
👇🏾Here’s my 2 cents on the #ctDNA #LiquidBiopsies @TheLancetOncol. Liquid biopsies are revolutionizing cancer care. Good to see it as integral tumor marker as opposed to a correlative. See thread 🧵 @OncoAlert more @myESMO #ESMO20 Will present #BESPOKE. https://t.co/3tBV7Huylr https://t.co/veBmsdd2Ez pic.twitter.com/lGXux4rin9
— Pashtoon Kasi MD, MS (@pashtoonkasi) September 16, 2020
HCPs shared that ctDNA sequencing is a welcome approach to the effective treatment of patients with immunotherapies. Being able to predict early response to immunotherapies was highlighted as a key outcome from the data shared by HCPs over the last few months.
Very elegant work using #ctDNA and circulating immune cell profiling to provide accurate, noninvasive, and early forecasting of therapeutic benefit for NSCLC patients receiving #immunotherapyhttps://t.co/Rh5EbsGxqL pic.twitter.com/8nv6sJ7Q9f
— soria (@jsoriamd) October 2, 2020
Continuing to share trial data, HCPs posted about how ctDNA could be used to guide chemotherapy treatment and encourage patients in their own treatment decisions. Other HCPs engaged in conversation with their peers about patient cases of treatment with chemotherapy citing the use of ctDNA as a potential option in the future to help guide the treatment approach.
Let me integrate this great discussion: initial stage operable? If yes, surgery; if not, chemo radiation can help to decrease tumor. ctDNA 🩸 🧬🧬pre chemo is a great idea to making decision, maybe in an early future
— Jose Fernando Moura, PhD (@FernandoOnco) August 26, 2020
In the online conversation of HCPs, we see that they are optimistic about the use of ctDNA in tailoring a patients treatment approach in a number of different stages of their cancer from early to much later stages.
ctDNA testing a controversial approach to cancer care
Even though the majority of HCPs have been sharing positively about the use of ctDNA to guide cancer care, there are others who are skeptical. Citing issues linked to cost and the necessity of adding yet another test to the already complicated environment, many HCPs had concerns about the tests and how translatable this will be in the larger practice setting. Dr Daniel Catenacci, an oncologist, in particular shared a number of these specific concerns in a conversation thread and commented on many of his colleague’s posts to provide his opinions.
The ‘Study’ you mention is asking the question whether physicians’ & patients’ choices regarding adj therapy will change based on the ctDNA results. It does not ask the question whether that tx change makes any difference, or more importantly, causes harm. 👉🏻That’s the problem.
— Daniel Catenacci (@DocCatenacci) September 19, 2020
To provide a balanced point of view HCPs discuss their experiences with liquid biopsy and in particular ctDNA. With the overwhelmingly positive news about ctDNA, these individuals warn of potential shortcomings when using these tests routinely and provide a more holistic view of testing in cancer care.
Absolutely. I’m a great supporter of liquid biopsy but I have found new T790M in a resected EGFRmut metastasis after twice testing negative on ctDNA.
— A/Prof Gavin Wright (@Clavikul) October 3, 2020
A new buzz word on the “tongues” of HCPs is financial toxicity. It is no surprise to see in this conversation among HCPs that they are discussing the cost that this additional testing will require from the patient. HCPs asked how a test like this would be covered financially and shared their experiences of how much it would cost a patient right now.
https://twitter.com/mraphaelmd/status/1307744846073954305
A long road ahead for ctDNA testing
In short, HCPs are optimistic and see the future value for patients but there is still a long road to travel on. HCPs highlight that more studies need to be done before they would be using this routinely and incurring the costs financially and in treatment success for patients.
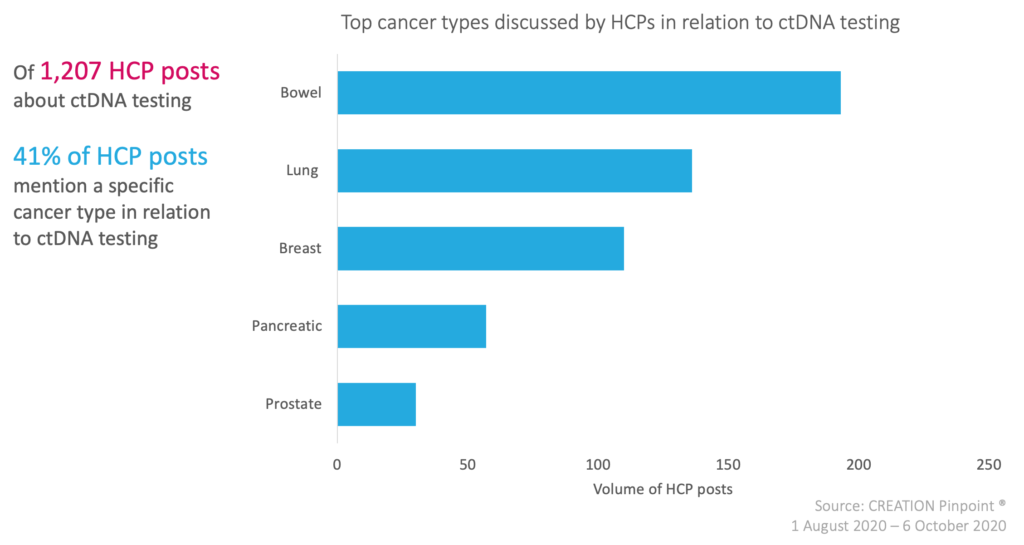
Over the time period studied, HCPs discussed ctDNA testing in all major cancers; breast, bowel, pancreatic, lung, and prostate to name a few. This type of testing could have a large reaching impact on the treatment of cancer patients in the future and HCPs are excited about the possibilities to take new ground in the fight against cancer.
To keep up to date with the latest HCP conversations about existing and developing treatment options, register for our monthly newsletter CREATION Knowledge.
![[VIDEO] Allyson Ocean on how and why social media empowers her work in #PancreaticCancer](https://creation.co/wp-content/uploads/2019/11/Screenshot-2021-11-04-at-10.47.05-704x528.png)
