Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout December 2021 we tracked the global conversations of 3,357 HCPs who posted 5,228 English-language Twitter posts about the launches and approvals of new products.
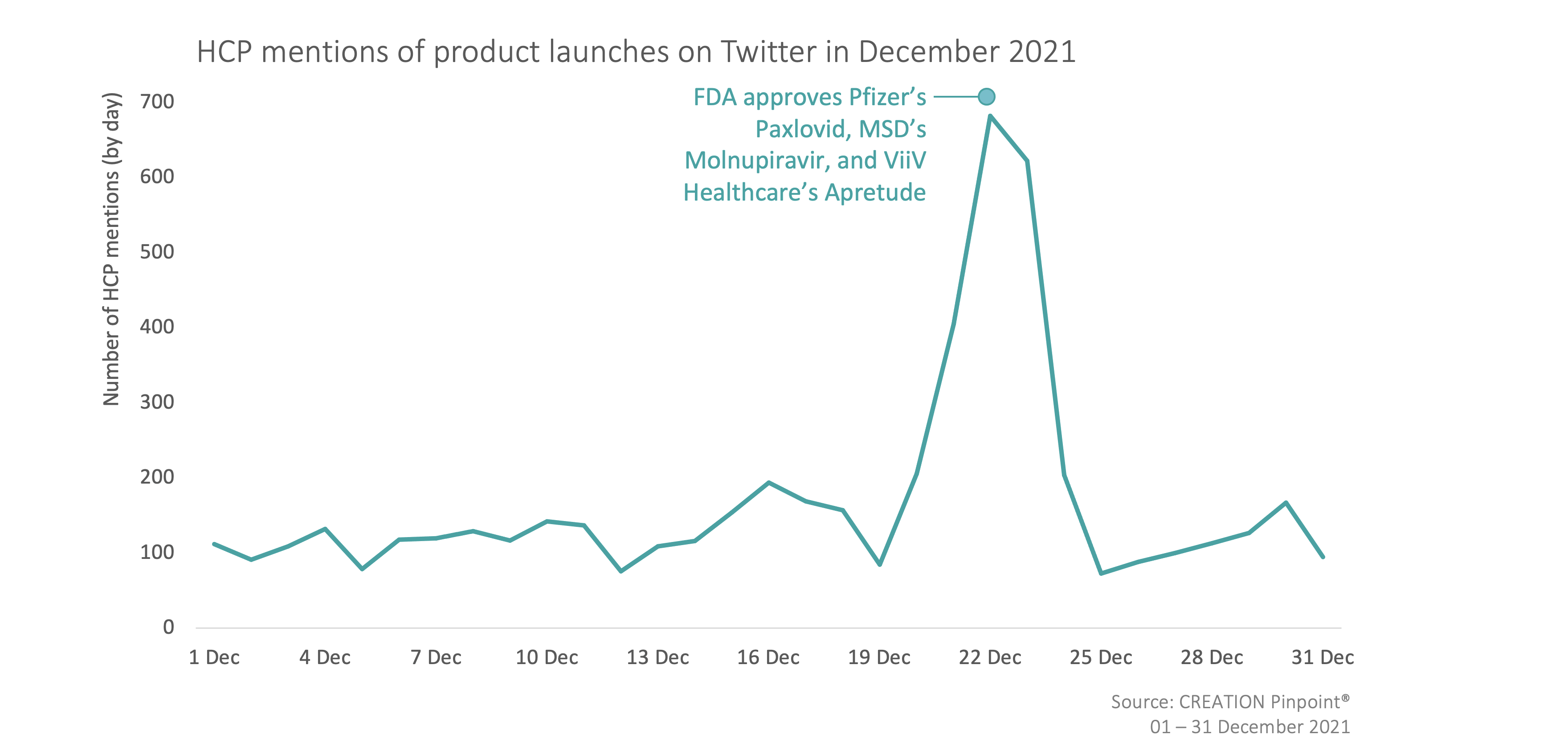
In December, HCPs discussed the approval of several treatments including three new COVID-19 treatments, an injectable alternative to daily pills for HIV prevention, and a small interfering RNA (siRNA) therapy to lower low-density lipoprotein cholesterol (LDL-C). The week leading up to Christmas saw a significant spike in conversation, mainly driven by HCPs sharing their thoughts on the FDA’s approval of two antiviral COVID-19 treatments for emergency use.
HCPs spoke positively of the approval of Pfizer’s Paxlovid (nirmatrelvir co-packaged with ritonavir) and MSD’s Molnupiravir, seeing both treatments as a step forward in the fight against COVID-19. However, some HCPs were restrained in their excitement, noting that widely available testing and free provision of the treatment would be required for these treatments to have a major impact.
.@US_FDA grants authorization to Pfizer's Paxlovid antiviral pill💊for COVID.https://t.co/VfZVT9Lj9t
BUT, IMPACT will be LIMITED if we don't have:
– free, widely available/accessible testing
– a way to provide treatment for free within 3-5 days of exposure/symptom onset— Céline Gounder, MD, ScM, FIDSA 🇺🇦 (@celinegounder) December 22, 2021
Looks like Paxlovid (and Molnupiravar) are getting FDA authorized tomorrow. It's an exciting step forward. If you're not familiar with why this is important and the issues surrounding these new anti-Covid pills, here are 2 recent essayshttps://t.co/qClag5KjUy https://t.co/C9oKhFBipw
— Eric Topol (@EricTopol) December 22, 2021
Earlier this month, HCPs also displayed excitement for another COVID-19 treatment, AstraZeneca’s Evusheld, which gained Emergency Use Authorization (EUA) from the FDA on 9 December. HCP’s heralded the monoclonal antibody treatment as a great alternative to vaccination for immunocompromised patients and those with a history of severe adverse reactions to a COVID-19 vaccine.
Excited re FDA approval of #AstraZeneca #Evusheld today
Great short-term #vaccine alternative for people who can't get or who won't respond to #COVID19 vaccines like transplant pts
Today spoke to one of my pts sheltering-in-place for >20 months. This gave her hope
Some pearls: pic.twitter.com/GZCENLSCt3
— Peter Chin-Hong MD (@PCH_SF) December 9, 2021
As well as discussing new COVID-19 treatments, HCPs showed an active interest in the FDA’s approval of ViiV Healthcare’s new treatment, Apretude (cabotegravir extended-release injectable suspension), for use in at-risk adults and adolescents to reduce the risk of HIV. As the first injectable treatment for HIV pre-exposure prophylaxis, HCPs marked it as an important milestone for preventing HIV without a daily pill.
.@US_FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention https://t.co/wxwI2BrlS8 The conclusion of a game changing @HIVptn study led by the amazing @doc_in_a_box Congratulations to the study team, investigators and study volunteers!
— Carlos del Rio (@CarlosdelRio7) December 21, 2021
Minding my own business getting slides ready for grand rounds and I see the FDA has approved injectable cabotegravir for HIV PrEP. I freakin LOVE this – the numbers of people who will be able to safely avoid HIV infection!!!
We need more wins like this. pic.twitter.com/RkFOWnygU3— Stella Safo, MD MPH (@AmmahStarr) December 21, 2021
Lastly, HCPs also welcomed the FDA’s approval of Novartis’ Leqvio (inclisiran) as the first and only siRNA therapy to lower LDL-C. Most HCPs were pleased with the news, however, some were critical of how long it took the FDA to approve the treatment.
Almost a year after the EMA approved inclisiran, the FDA now grants inclisiran approval in the US. Exciting news for patients and colleagues in the US. Now the implementation begins. Glad to have been part of the journey
— Prof Kausik Ray FMedSci (@ProfKausikRay) December 23, 2021
The three most shared links from HCPs discussing product launches in December were:
- An FDA article about the emergency use authorization of Paxlovid
- An FDA article about the approval of the first injectable treatment for HIV pre-exposure prevention
- An FDA article about the emergency use authorization of Molnupiravir
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 December and 31 December 2021 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 December and 31 December 2021, there were 5,228 HCP mentions of new pharmaceutical product launches and drug approvals from 3,357 unique HCP authors from around the world.
View the latest product launch and archived trackers here

 By
Paul Cranston and Tomi Shobande
By
Paul Cranston and Tomi Shobande 


