Every month, CREATION.co’s tracking updates bring you the latest insights from the online conversation of healthcare professionals (eHCPs) across the globe discussing product launches. Discover which new drug approvals eHCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout September 2023 CREATION.co tracked the global conversations of 1,555 eHCPs who posted 2,379 English-language X (formerly Twitter) posts about the launches and approvals of new products.
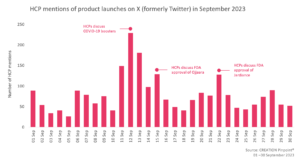
In September, fewer eHCPs discussed treatment approvals compared to the previous month and there were 14% less product launch mentions. eHCPs offered their thoughts on the approval of the new COVID-19 mRNA vaccine boosters as well as the approval of Jardiance for the treatment of chronic kidney disease.
On 11 September, the FDA granted full approval and authorisation for emergency use of the updated mRNA COVID-19 vaccines boosters, by Moderna and Pfizer, formulated to more closely target currently circulating variants. The reactions of eHCPs to the approval news were quite mixed. A number of eHCPs discussed, in their opinion, the lack of an emergency for such an approval, and raised concerns around the possible side effects on some population groups. On the other hand, many eHCPs recommended the booster vaccine, highlighting the severity of the disease and the importance of vaccinations to avoid hospitalisations.
FDA approved the updated booster! It just can't come soon enough 👇🏻
https://t.co/Q0bKFMitgh— 🕊Dr. Natalia 🌻🪬 (@SolNataMD) September 11, 2023
ICYMI: the updated covid-19 booster has been approved, with CDC approval expected soon.
Have questions? PLEASE ASK ME!
Covid19 kills. The flu kills. Vaccines work to prevent death. And they are safe.
I get vaccinated; and vaccinate my children.https://t.co/aZi8grFLZp— Meena Bewtra (@DrsMeena) September 12, 2023
On 22 September, the FDA announced the approval of Jardiance (empagliflozin) by Eli Lilly and Boehringer Ingelheim, an SGLT2 inhibitor, to reduce the risk of sustained decline in estimated glomerular filtration rate (eGFR) and hospitalisation in adults with chronic kidney disease (CKD). eHCPs did not express many opinions around the approval but they frequently shared the news of the approval online, often sharing the announcement of Boehringer Ingelheim.
https://twitter.com/tannercorse/status/1705664727932682467
On 15 September, the FDA granted approval to GSK’s Ojjaara (momelotinib) for the treatment of patients with intermediate or high-risk myelofibrosis with anaemia. Ojjaara is a once-a-day, oral JAK1/JAK2 and activin A receptor type 1 (ACVR1) inhibitor. eHCPs were deeply excited, as they quoted, about the approval having the potential to change the clinical practice. They mentioned that they are eager to have this fantastic news brought into practice in the UK and EU as well.
#ICYMI: With the approval of momelotinib by the FDA, the updated use for its role in myelofibrosis will potentially change clinical practice, according to Ruben Mesa, MD. @mpdrc https://t.co/3bjjS0hgcR
— CancerNetwork® (@CancerNetwrk) September 25, 2023
Deeply excited by the FDA approval of Momelotinib for patients with myelofibrosis with anemia, grateful for all selfless participation of patients and #MPN colleagues that led to this advance. @LevineCancer @WakeCancer @myMPNRF https://t.co/A8fW0NROoT
— Ruben A. Mesa, MD (@mpdrc) September 15, 2023
The three most shared stories from eHCPs discussing product launches in September were:
- A FDA press release on the approval of the updated mRNA COVID-19 vaccine boosters.
- A NEJM article on The Accelerated Approval Program for Cancer Drugs — Finding the Right Balance.
- A GSK press release on the approval of Ojjaara for myelofibrosis patients with anaemia.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language X conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 September and 30 September 2023 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their X conversations.
- Between 1 September and 30 September 2023, there were 2,379 eHCP mentions of new pharmaceutical product launches and drug approvals from 1,555 unique eHCP authors from around the world.
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou 
