Every month, CREATION.co’s tracking updates bring you the latest insights from the online conversation of healthcare professionals (eHCPs) across the globe discussing product launches. Discover which new drug approvals eHCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout October 2023 CREATION.co tracked the global conversations of 1,467 eHCPs who posted 2,088 English-language X (formerly Twitter) posts about the launches and approvals of new products.
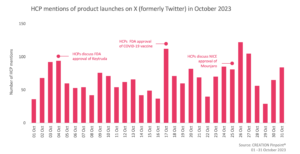
In October, fewer eHCPs discussed treatment approvals compared to the previous month and there were 13% less product launch mentions.
On October 3, the FDA announced the approval of Novavax’s updated COVID-19 vaccine, formulated to better protect against currently circulating variants. eHCPs responded positively to the approval, calling it “awesome news” and an effective yet different option, for vaccination against COVID-19. Prof. Wasim Hanif expressed his support for the new vaccine stating that it is more effective and possibly presents with fewer side effects compared to other COVID-19 vaccines.
Novavax COVID-19 vaccine finally approved today. Lots of immune compromised people can't medically get mRNA vaccines. Spread the word.
find novavax near you: https://t.co/LcPiczc4Lohttps://t.co/UuOpweJw3R
— WEAR A MASK 🇵🇸😷🇸🇩 (@WeKeepUs_Safe) October 3, 2023
A great week for vaccines:
-mRNA vaccine researchers win Nobel Prize
– second malaria vaccine approved by WHO
– Novavax COVID-19 vaccine approved by US FDA
— Dr. Lucky Tran (@luckytran) October 3, 2023
On 16 October, the FDA approved Merck MSD’s Keytruda (pembrolizumab) for adjuvant, neoadjuvant treatment of resectable non-small cell lung cancer (NSCLC). The majority of eHCPs who discussed the approval, did not share any positive or negative opinions though a small number of eHCPs showcased their excitement as they felt it offers another option for NSCLC patients and showcased promising results during the Keynote 671 study.
FDA approves first perioperative immunotherapy regimen for resectable NSCLC. Based on KEYNOTE 671, #neoadjuvant pembrolizumab + chemotherapy followed by one year of adjuvant pembrolizumab now FDA approved for pts with resectable (T≥4 cm or N+) NSCLC.https://t.co/wsSIoJAJG4
— Stephen V Liu, MD (@StephenVLiu) October 17, 2023
#ESMO23 Dr. @DoctorJSpicer presents OS data from KEYNOTE 671: neoadjuvant pembrolizumab + chemotherapy followed by surgery and adjuvant pembro x 1 year. Perioperative IO improves survival. OS HR 0.72, 3y OS rate 71% vs 64%, impressive tails. Now an FDA approved standard. pic.twitter.com/EKRAeSV8oO
— Stephen V Liu, MD (@StephenVLiu) October 20, 2023
On 25 October, NICE published guidelines on the use of Eli Lilly’s Mounjaro (tirzapetide) for the treatment of type 2 diabetes as it became available to the NHS. A number of eHCPs shared the guidelines with their peers and commented on how it will tackle the problem of GLP-1 shortages that the NHS was facing. eHCPs were pleased with the approval quoting “a new era of multi incretins begins”
It’s out .. NICE TA Tirzepatide … the era of the multi incretin begins … https://t.co/TlnjWAAI8H pic.twitter.com/bW1LAqVHOn
— Hannah Beba (@HannahBeba) October 25, 2023
🚨 #Tirzepatide / #Mounjaro 🚨
🔸will be available on @NHSuk
🔹 initially for #type2diabetes
If:
➡️ triple therapy ineffective/not indicated ➕
➡️ BMI >35 (ethnicity adjusted)
Or
➡️BMI < 35 with certain criteria👏🏻👏🏻👏🏻 @NICEComms @parthaskar
🔗 https://t.co/AaMlzArR2b pic.twitter.com/YcECXM9js5
— Patrick Holmes (@drpatrickholmes) October 25, 2023
The three most shared stories from eHCPs discussing product launches in October were:
- A FDA press release on the approval of the updated Novavax COVID-19 vaccine.
- An BusinessWire article on the FDA having denied the sNDA for patisiran for an indication for ATTR-CM.
- A NICE press release on the approval of tirzapetide for treating Type 2 Diabetes.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language X conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 October and 31 October 2023 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their X conversations.
- Between 1 October and 31 October 2023, there were 2,088 eHCP mentions of new pharmaceutical product launches and drug approvals from 1,467 unique eHCP authors from around the world.
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou