11.08.2023 | Insight
Online conversations suggest US HCPs are not yet convinced about Daiichi Sankyo’s Vanflyta
Last month, the FDA approved the use of Vanflyta, an FTL3 inhibitor (FTL3i) used as a treatment for patients with newly diagnosed FLT3-ITD positive acute myeloid leukaemia (AML), four years after Daiichi Sankyo first applied for the use of their drug in the US market. The FDA also approved the Leukostrat CDx FLT3 Mutation Assay, a diagnostic test for the FTL3 mutation used to identify patients who are eligible for the use of FTL3i.
Vanflyta is not the first FTL3i in the US market – it is competing against Rydapt (Novartis received FDA approval in 2017) and Xospata (Astellas received FDA approval in 2018). This begs the question, what do online healthcare professionals (eHCPs) think about the approval of Vanflyta in the US?
Using CREATION Pinpoint® we analysed over 1,000 posts in the unprompted online conversation about FTL3 inhibitors between 1st June 2022 and 31st July 2023. Almost 50% of this online conversation came from HCPs in the US, the country impacted by the approval.
QUANTUM-First trial data created excitement among eHCPs
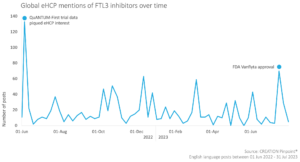
Within the online FTL3i conversation, the majority of eHCP posts were shares of content posted by other accounts, such as scientific journals and news outlets. Only 15% of the overall eHCP conversation comprised original posts from eHCPs, who were mostly talking about research and trial data. eHCPs were keen to share updates from sources that they trust, such as the Blood Journal on X (formerly known as Twitter).
As shown on the graph above, eHCPs were most actively discussing FTL3 inhibitors on 11 June, 2022. 62% of the conversation on this day was focused on the QuANTUM-First clinical trial on quizartinib (branded as Vanflyta), after the trial data was released at the European Hematology Association congress. Astellas’ ADMIRAL trial (investigating gilteritinib, branded as Xospata) was also mentioned, but generated significantly less excitement among eHCPs, with only 20% of eHCP posts in the FTL3i conversation mentioning it over the period of the EHA congress.
#EHA22 #leusm Erba: Quizartinib QUANTUM-First Ph3 RCT (7+3 +/- Quiz days 8-21) for FLT3-ITD+ AML. Age 18-75. Quiz is type II FLT3i only active against FLT3-ITD. Up to 3yr Quiz maintenance permitted. N=265v268 pic.twitter.com/lY5jlKXdRO
— Aaron Logan, MD, PhD, MPhil (@hemedoc) June 11, 2022
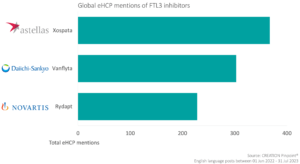
eHCP product conversation focus was on Xospata until July 2023, when attention turned to Vanflyta
While data on Vanflyta was the main focus of eHCP conversation over the EHA congress, Astellas’ FTL3i Xospata was the focus of 46% of the product-related eHCP conversation in our dataset over the time period. eHCPs were keen to discuss trial data on Xospata, with a mixture of responses to the data. Miguel Perales posted that the results of the trial were “disappointing” and reflected that the results illustrate the importance of phase 3 trials, which was shared by 17 other eHCPs.
While earlier conversations about Vanflyta had included concerns about the control arm in trials, more recently eHCPs have highlighted the effectiveness of Vanflyta, citing recent research.
Quizartinib is a good option for AML patients with FLT3-ITD mutations@Innov_Medicine @The_InnovationJ
Quizartinib is a novel, effective, and generally well-tolerated therapeutic option for adult AML patients with FLT3-ITD mutations. With potent FLT3 inhibitors incorporated… pic.twitter.com/296CABg9Yu
— The Innovation | Medicine (@Innov_Medicine) July 16, 2023
With the FDA approval of Vanflyta announced on 20 July, 2023, it does not come as a surprise that 22% of the eHCP mentions of the drug in our dataset were posted on or after 20 July, when eHCPs were posting and sharing information on the FDA approval, with some also enthusing that ”quizartinib boosts AML survival”.
#Quizartinib boosts #AML survival, regardless of SCT
👉https://t.co/3NExdI1sll@MDedgeHemOnc pic.twitter.com/0yKcxCfBwj— David Henry (@davidhenrymd) July 21, 2023
Japanese eHCPs were excited about the 2019 launch of Vanflyta in Japan
eHCPs in Japan, where Vanflyta was approved in 2019, were excited about the drug, with one oncologist describing it as “super cool” in 2018 when talking about the OS achieved in the QuANTUM-First trial data. Other eHCPs in Japan more recently shared education on how to use the treatment and news articles on the approval of Vanflyta in the US.
「国際共同第III相試験QuANTUM-RでOS延長を示したFLT3阻害薬キザルチニブ」って、音の響きだけで超かっこいいな(厨二病)
— レ点🧬💉💊 (@m0370) September 11, 2018
Lack of access is a point of frustration within US eHCP conversations
Although eHPCs in the US have been actively discussing Vanflyta online, only one eHCP mentioned Daiichi Sankyo’s diagnostic test, Leukostrat CDx FLT3 mutation assay, at the time of this research. Matthew Mei, a haematologist in California, mentioned the diagnostic test in a discussion thread on the social media platform X about problems some HCPs are finding with access in the US. In the same thread, another haematologist – Chadi Nabhan from Chicago – made the statement that “Access in the US is always an issue”.
Normal karyotype, no NGS, FLT3 ITD, no allelic ratio, no access to FLT3 inhibitors.
— Andres Gomez (@GomezDLeonMD) December 27, 2022
With that being said, eHCPs use social media as a network to collaborate with each other and ask advice on how to treat their patients. One eHCP from India used X to ask his peers if they had any experience with midostaurin when looking at treatment options for his patient in an area outside of AML with the FTL3 gene mutation.
As with all diseases, the journey to access the right treatment can only begin following accurate diagnosis. In the AML space, we have observed among eHCPs a disconnect in the level of awareness between new treatment developments and a new diagnostic technique which could help cancer patients access that treatment faster.
Especially within AML, a cancer type in which no screening tests have been shown to be helpful in finding it early, there is an opportunity for other HCPs, patients, patient groups and pharmaceuticals to help raise awareness and circulate education resources to raise the profile of Leukostrat CDx assay.
Keep up to date with eHCPs’ reactions to new product approvals through our monthly Product Approval Tracker update. To find out more about their views in AML or in any health topic please get in touch, we’d love to connect.
Methodology
This article analysed the conversation on 445 of HCPs in the globally discussing FTL3 inhibitors and related terms between 01 June 2022 and 31 July 2023 using CREATION Pinpoint®.
 By Hannah Ghinn
By Hannah Ghinn