Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
Throughout July 2023 we tracked the global conversations of 2,037 eHCPs who posted 2,882 English-language X posts about the launches and approvals of new products.
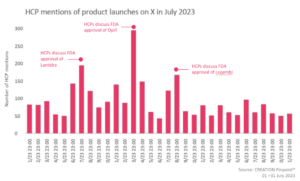
In July, several product approvals were discussed online by HCPs. They offered their thoughts on Perrigo’s Opill, the first ever non prescription daily oral contraceptive as well as expressed their views around Lantidra, the first cellular therapy to treat patients with Type 1 Diabetes.
During July, the FDA granted full approval to Eisai’s Leqembi (lecanemab-irmb) indicated for patients with Alzheimer’s disease. Leqembi is the first amyloid beta-directed antibody to be converted from an accelerated approval to a traditional approval for the treatment of Alzheimer’s disease. eHCPs had mixed reactions regarding the approvals with certain eHCPs being delighted by it, calling it “another hopeful, positive step forward in the fight against Alzheimers disease”, while others questioned its efficacy and pricing. An eHCP mentioned that Leqembi does not stabilise the condition and another called it inaccessible for many due to its price point.
Potentially great news for Alzheimer's patients! #MedTwitter #Medicine #Health #Care https://t.co/Khn83YojAw
— Dr. Jason J. Begalke 🕊️ (@DrJasonBegalke) July 15, 2023
I am a physician specializing in dementia care. FDA has now approved lecanemab (Leqembi) for Alzheimer's. It <reduces the rate of decline> of brain function by a little bit. The burden of treatment is high. I will NOT proactively offer it but … [cont'd]
— J. P. Roberts 🇺🇦 (@jpr602) July 7, 2023
On 20 June, the FDA announced the approval of CellTrans Lantidra, the first allogeneic pancreatic islet cellular therapy, for the treatment of type 1 diabetes patients who get frequent severe hypoglycaemia. eHCPs spread the approval news calling the new treatment ‘revolutionary’ and potentially a big benefit of the treatment being the potential freedom from pricks/pumps. A significant number of eHCPs were excited to see the treatment being implemented and developed.
Interesting news in the world of #T1Diabetes
Approval from @US_FDA
That side effect profile …isn’t insignificant though
Will be intriguing to see this develop #gbdoc https://t.co/bb5DZTzWVV pic.twitter.com/v4o2ddJveY
— Partha S Kar 🇮🇳🇬🇧🏏🎥 (@parthaskar) July 6, 2023
What are the benefits ?
In the pre-approval study , 21 out of 30 patients went off insulin injections for an year. 4/30 couldn’t go off insulin for even a day – but their requirements reduced.
Big benefit – potential freedom from pricks/ pumps
— Karthik Balachandran (@karthik2k2) July 23, 2023
On 15 June, the FDA granted accelerated approval to Opill (norgestrel) tablets for over the counter use as the first daily oral contraceptive. The approval of this progestin-only oral contraceptive pill raised many positive reactions with the eHCPs calling the approval a “big step forward for US public health” and a “milestone that could significantly expand access to contraception”. eHCPs celebrated the approval as positive news for womens’ health and autonomy of reproductive health.
Historic news! The FDA approved over-the-counter sales of Opill birth control pills!
This moment is thanks to decades of hard work and research. Congratulations @freethepill @IbisRH @AdvocatesTweets and everyone else who made today possible! #FreeThePillhttps://t.co/RqgJIOX3Ra
— Dr. Daniel Grossman (@DrDGrossman) July 13, 2023
Today, the FDA has approved the first over-the-counter birth control pill––a major step forward for US public health and bodily autonomy. Two months ago, I wrote an essay for the @nytimes about why this matters for reasons far beyond reproductive health alone. https://t.co/U6lkE9mp5s
— Eric Reinhart (@_Eric_Reinhart) July 13, 2023
On 17 July the FDA approved AstraZeneca and Sanofi’s Beyfortus (nirsevimab-alip) for the prevention of Respiratory Syncytial Virus (RSV) in infants and children up to 24 months old at risk for infection, as a single injection. eHCPs shared the approval news as a new treatment option entered the space, being “good news” and “a major step in preventing RSV”.
Important new FDA approval for RSV prevention in newborns/young children – important new option https://t.co/5Pcl3rDM1x
— Steven Pergam, MD, MPH (@PergamIC) July 17, 2023
Good news today @FDA approves @Nirsevimab for @RSV prevention in children @FloridaAAP @GAChapterAAP @Alchapaap @UFHealthJax @WolfsonChildren @BaptistHealthJx @UFMedicineJax @UFHealth https://t.co/9pH77CRNvm
— Mobeen Rathore, MD, CPE, FAAP, FPIDS, FIDSA, FSHEA (@mhrathore) July 17, 2023
The three most shared links from HCPs discussing product launches in June were:
- A Nature review article on FDA approval of the first cell therapy of Type 1 Diabetes.
- An FDA news release on the approval of the first Nonprescription Daily Oral Contraceptive.
- An FDA news release on the approval of a new RSV treatment for infants and toddlers.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language X conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 July and 31 July 2023 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their X conversations.
- Between 1 July and 31 July 2023, there were 2,882 eHCP mentions of new pharmaceutical product launches and drug approvals from 2,037 unique eHCP authors from around the world.
Click here to read the latest Product Launch Tracker
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou 

