Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout July 2022 we tracked the global conversations of 1,836 HCPs who posted 2,413 English-language Twitter posts about the launches and approvals of new products.
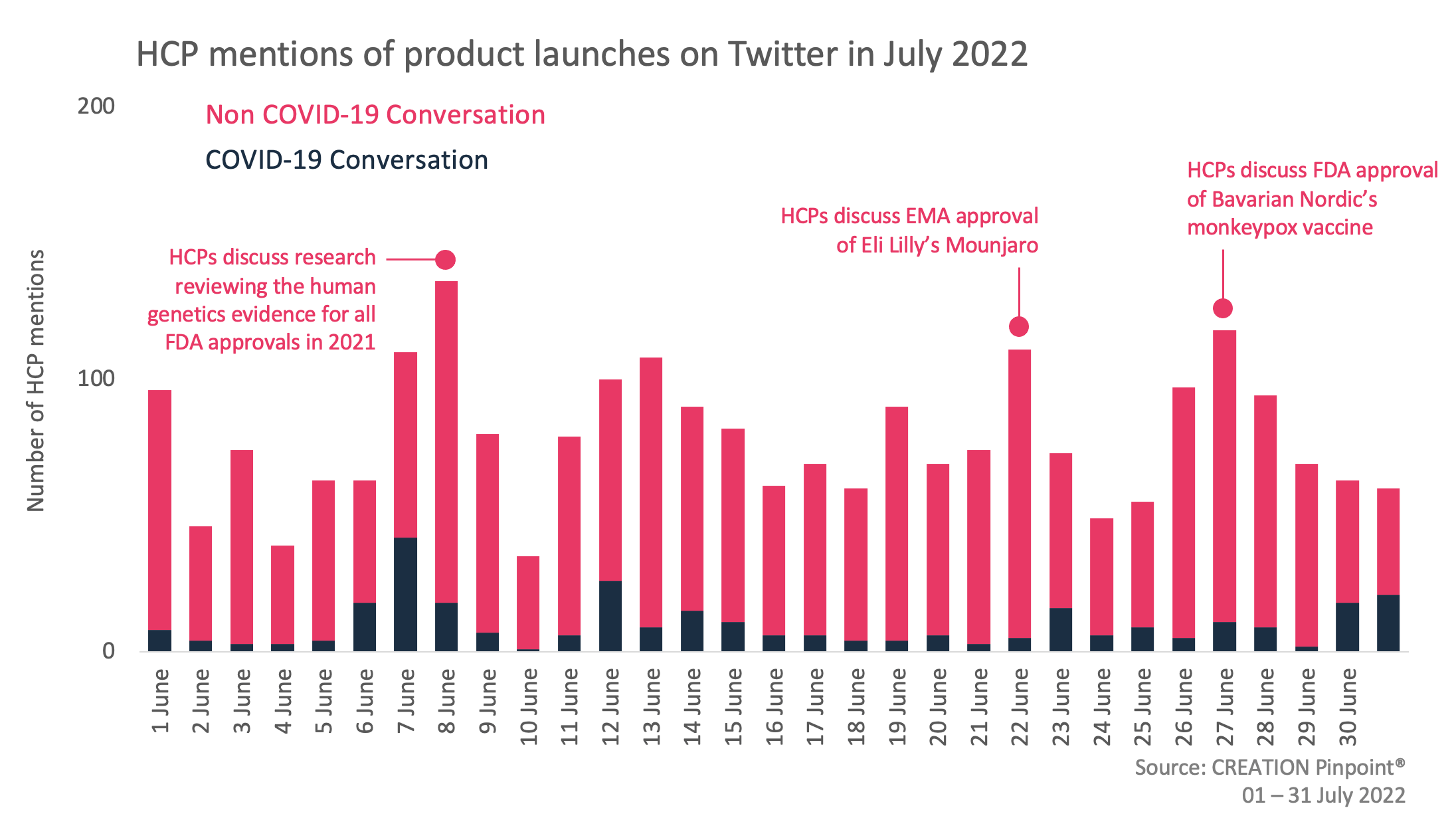
Across the month of July, HCPs commented on and shared the news of several product approvals across various indications including type II diabetes, vitiligo and ALK-positive tumours. Additionally, HCPs also held discussions around the approval of treatments for monkeypox, as well as a recently published review of all newly approved FDA treatments from 2021, assessing the human genetics evidence for each approval.
The recent outbreak of monkeypox currently affecting many countries has led to HCPs discussing regulatory bodies stances on several treatment options for the disease. Initially on the 14th July several HCPs made comments on the lack of availability or access to TPOXX (tecovirimat) for those suffering from monkeypox, despite it already being an FDA approved drug.
Now that @US_FDA has expedited release of #monkeypox vax from Europe, @relucantlyjoe & I want to improve access to another priority intervention: treatment. #monkeypox patients are suffering, even though there’s a drug in the US that may help them.https://t.co/jmGWpJgk6Q
/1
— Jay Varma (@DrJayVarma) July 15, 2022
Later in the month, 19 HCPs also shared the FDA’s news that they had approved a supplement to the biologics licence for the Jynneos vaccine, manufactured by Bavarian Nordic, in order to allow for increased manufacturing of the monkeypox vaccine.
Today, the FDA is announcing it has approved a supplement to the biologics license for the JYNNEOS Vaccine, which is approved to prevent smallpox and monkeypox, to allow for additional manufacturing capabilities at one of the plants where the vaccine is made.
— U.S. FDA (@US_FDA) July 27, 2022
HCPs also shared the approval of Incyte’s Opzelura (ruxolitinib) cream for the treatment of non segmental vitiligo in adults and paediatric patients 12 years of age and older. Opzelura is the first ever home drug that can reverse skin pigmentation. HCPs were positive about the approval and described it as exciting progress for the therapy area.
#vitiligo Rx breaking news : an FDA approved treatment opzelura is finally here 👏👏#vitiligotreatment #FDA https://t.co/jMNUn7o7Lo
— Aisha Sethi (@SethiAisha) July 19, 2022
We now have an approved treatment for repigmentation in vitiligo! Topical ruxolitinib cream was FDA approved yesterday – such exciting progress! @tcelltracker @HarrisVitiligo https://t.co/D6xVJun2n3
— William Damsky MD, PhD (@billdamsky) July 19, 2022
Another approval which caught HCPs attention in July was Xalkori (crizotinib, Pfizer), which the FDA approved for adult and paediatric patients with unresectable, recurrent, or refractory inflammatory ALK-positive myofibroblastic tumours (IMT). When sharing the news of this approval HCPs chose to draw attention to the journey the drug had taken from the CREATE trial four years ago to its recent approval; while others celebrated the power of precision medicine demonstrated by the approval.
Crizotinib finally approved by FDA for ALK-positive inflammatory myofibroblastic tumor, four years after publishing the prospective EORTC Phase 2 90101 trial CREATE (DOI: 10.1016/S2231-2600(18)30116-4 and an update in 2021 (DOI: 1016/j.ejca.2021.07.016) pic.twitter.com/5LGoE7VjrL
— Patrick Schöffski (@schoffski) July 17, 2022
🎯Wow ! Power of #precisionmedicine in ultra-rare disease leads to FDA approval 👉🏼Crizotinib in ALK+ IMT !!!
✅12/14 pediatric patients had objective responses
✅5/7 adult patients had objective responses 🎯🧬https://t.co/uY4vhmgo4k pic.twitter.com/NwDpHx6DZz— Vivek Subbiah, MD (@VivekSubbiah) July 16, 2022
Additionally, HCPs also shared the marketing authorisation granted by the European Commission for Mounjaro, a treatment for adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise. Endocrinologist Cristobal Morales played a key role in sharing the news of this approval, with 21 HCPs sharing his post.
https://t.co/lBJqlLoOHN Últimas noticias🗞🗞“Mounjaro is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise” En Europa🇪🇺 #Tirzepatide @LillyTrials @SEDiabetes @sociedadSEEN @estebanjodar @ValleAlfonso pic.twitter.com/dWI41Zs0ZG
— Cristobal Morales (@CristobMorales) July 22, 2022
Finally, HCPs also discussed the success of genetics informed drug discovery. One of the tenets of “personalised medicine” is that medications will be tailored to individuals based on their personalised genetics. Last year, the U.S. Food and Drug Administration’s Centre for Drug Evaluation and Research approved 50 drugs. A new study found that 33, or 66% of the approved drugs, were supported with genomic data, a statistic which many HCPs were impressed with.
Human genetics evidence supports TWO-THIRDS (!!!) of the 2021 FDA-approved drugs https://t.co/rwtobDjutz @NatRevDrugDisc
— Katalin Susztak (@KSusztak) July 8, 2022
This is quite an incredible statistic. And really encouraging for the ultimate success of genetics-informed drug discovery.
Human genetics evidence supports two-thirds of the 2021 FDA-approved drugs https://t.co/sqMxM1xdvn
— euan ashley (@euanashley) July 12, 2022
The three most shared links from HCPs discussing product launches in June were:
- A Nature article reviewing the human genetics evidence to support all FDA approvals in 2021
- An EMA article outlining their recommendation of Mounjaro for the treatment of type 2 diabetes mellitus
- A STAT news article entitled ‘Monkeypox patients should not be left to suffer when an FDA-approved drug could help’
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 July and 31 July 2022 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 July and 31 July 2022, there were 2,413 HCP mentions of new pharmaceutical product launches and drug approvals from 1,836 unique HCP authors from around the world.
Click here to read the latest article

 By
Paul Cranston and Tomi Shobande
By
Paul Cranston and Tomi Shobande 



