12.03.2024 | Tracker
Product Launch Tracker: eHCPs are enthusiastic about the first cellular therapy for advanced melanoma.
Every month, CREATION.co’s tracking updates bring you the latest insights from the online conversation of healthcare professionals (eHCPs) across the globe discussing product launches. Discover which new drug approvals eHCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout February 2024 CREATION.co tracked the global conversations of 1,346 eHCPs who posted 1,955 English-language X (formerly Twitter) posts about the launches and approvals of new products.
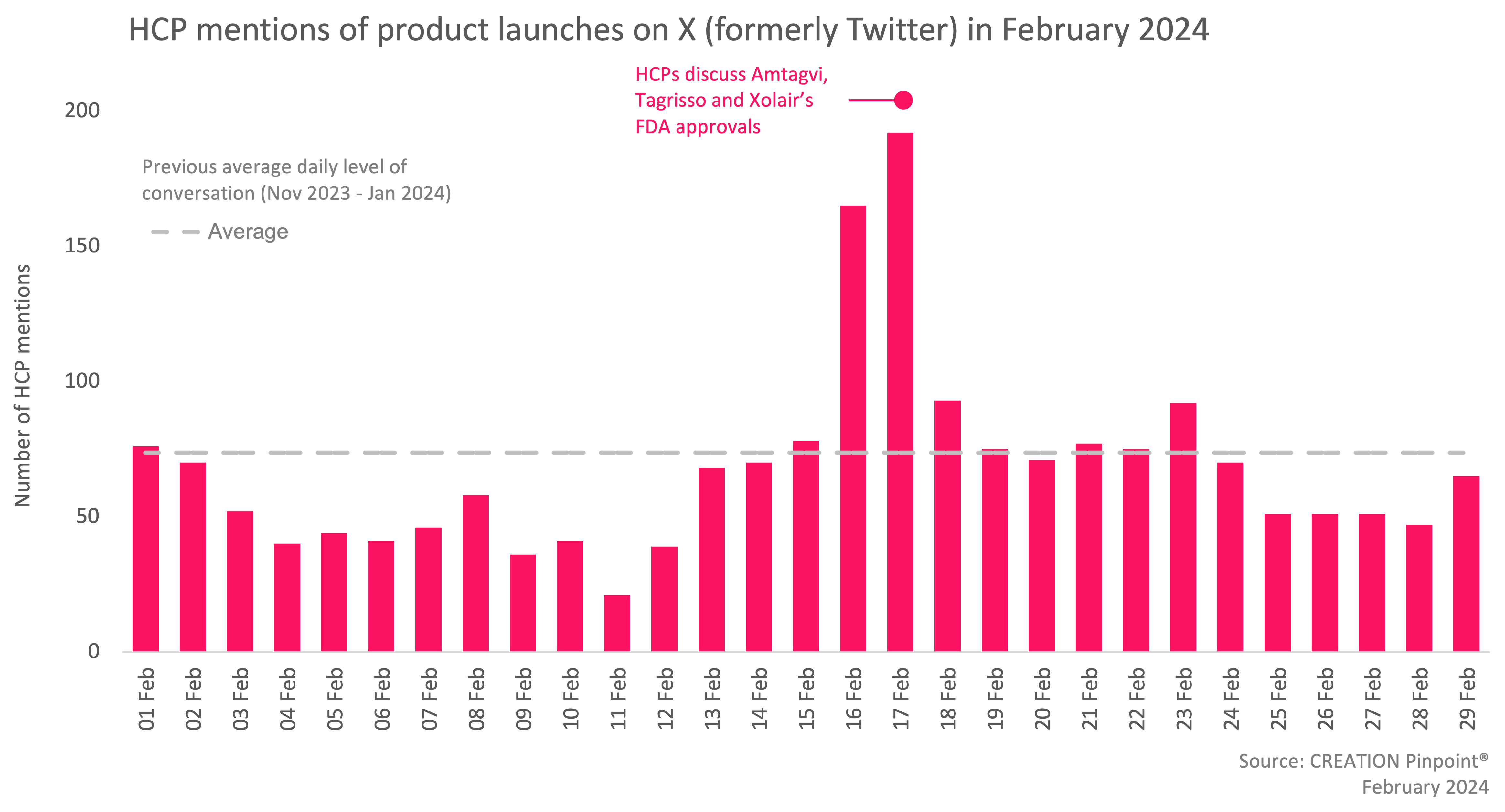
In February, 160 more eHCPs discussed treatment approvals compared to January and there were 19% more eHCP mentions of new pharmaceutical product launches and drug approvals.
On 16 February, the FDA approved Iovance Biotherapeutics’ Amtagvi (lifileucel), the first cellular therapy to treat patients with unresectable or metastatic melanoma. eHCPs were thrilled about the approval, calling it a “major milestone” and a “genuine game changer” in the solid tumour therapy area. Tens of eHCPs, including Dr Ian Weissman, characterised the treatment as groundbreaking, as it shrank tumours in a third of the patients who had run out of treatments. Among other eHCPs, Dr Patrick Hanley congratulated the team behind this medical advancement.
Lifileucel (TIL cell therapy from @IovanceBio) is now FDA approved for advanced PD-1 refractory melanoma!!! The melanoma community is so grateful to the patients, caregivers, and clinicians who have made the clinical trials of this therapy possible and got lifileucel to approval.… pic.twitter.com/y7FiyAYJ4Q
— Allison Betof Warner, MD, PhD (@DrBetofMDPhD) February 16, 2024
big day in solid tumor oncology– lifileucel has gained FDA approval as the first cellular therapy for melanoma!! excited for more patients to have access to TIL soon, and to build on this milestone going forward @ASTCT @CureMelanoma @AIMatMelanoma @MSKCancerCenter
— Jim Smithy (@jsmithymd) February 16, 2024
On the same day, the FDA approved AstraZeneca’s Tagrisso (osimertinib) combined with chemotherapy for patients with EGFR-mutated non-small cell lung cancer.
A few eHCPs took into account some of the advantages that the treatment can offer, such as significant progression-free survival benefits. However, more eHCPs were critical of the approval stating that “the FDA has no standards” and that “they are permitting an option that could worsen outcomes for people”. This negative sentiment was also present because overall survival data were considered not mature enough and physicians suggested the treatment had an unclear impact on survival.
Dr Estela Rodruguez expressed her opinion stating that sometimes the FDA approves treatments that as a doctor, she is not ready to start in all patients”.
FDA approves FLAURA 2: first line osimertinib plus chemotherapy for advanced #EGFR NSCLC. Adding chemotherapy improved PFS (HR 0.62) but at the cost of toxicity and infusions every 3 weeks. Unclear impact on survival. More benefit in those with mets?https://t.co/vlZWkpxoi6
— Stephen V Liu, MD (@StephenVLiu) February 16, 2024
FDA has no standards
Approving chemo + Osi based on FLAURA2 is a terrible decision
Osi -> chemo may have similar or better OS with better QoL.FDA has no clue. They are permitting an option that could worsen outcomes for people.
Terrible decision, par for course pic.twitter.com/bMgYZk6W9T— Vinay Prasad MD MPH (@VPrasadMDMPH) February 16, 2024
Also on 16 February, the FDA approved Genentech’s Xolair (omalizumab) injection as the first medication to help reduce allergic reactions to multiple foods after accidental exposure. eHCPs were thrilled about this approval and characterised it as a “very exciting announcement”. Dr Jual Ivancevich posted online stating that “this groundbreaking approval provides new hope for those affected”. eHCPs also congratulated the team who contributed to creating this treatment.
Exciting news for food allergy!
FDA approves Xolair as first and only medicine for children and adults with one or more food allergies https://t.co/BhSsjqcXji
— Dr Katherine Anagnostou (@PedAllergyDoc) February 16, 2024
Food Allergies Could Become Less Scary With New Drug https://t.co/Mm3DTu3hAW
— Amy Kubal (@AmykRd) February 22, 2024
The three most shared stories from eHCPs discussing product launches in February were:
- An Iovance Biotherapeutics press release on the approval of Amtagvi for advanced melanoma.
- An FDA press release on the approval of Tagrisso for non-small cell lung cancer.
- An FDA press release on the approval of Amtagvi for advanced melanoma.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language X conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 February and 29 February 2024 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their X conversations.
- Between 1 February and 29 February 2024, there were 1,346 eHCP mentions of new pharmaceutical product launches and drug approvals from 1,955 unique eHCP authors from around the world.
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou