Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
Throughout January 2023 we tracked the global conversations of 1,954 HCPs who posted 2,916 English-language Twitter posts about the launches and approvals of new products.
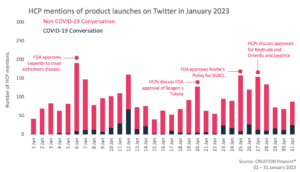
In January, several product approvals were discussed by online HCPs, with them offering their thoughts on a new treatment for Alzheimer’s disease and several newly approved cancer products, from companies including Roche, Merck and Eli Lilly.
One of the first approvals which generated significant conversation among HCPs was the FDA approval of Eisai’s Leqembi (lecanemab-irmb) for Alzheimer’s disease, via the accelerated approval pathway. Leqembi is the second of a new category of medications approved for Alzheimer’s disease that target the fundamental pathophysiology of the disease. HCPs opinions on this were significantly split, with some seeing the approval as an important step forwards in reducing the suffering caused by the disease, while others felt more cautious about the accelerated-approval due to the unproven surrogate endpoint and unanswered questions about its potential tie to patient harm.
HUGE NEWS! @US_FDA approves #Alzheimers drug shown to moderately slow cognitive decline in early disease stages. While more advances are urgently needed, this represents a solid step towards reducing suffering from this dreaded disease. 👏https://t.co/jNg2FiRfUc via @statnews
— Dr. Ron DePinho (@RonDePinho) January 6, 2023
Although not unexpected, what a shame that @US_FDA approved #lecanemab (Leqembi) through the accelerated approval pathway based on the same unproven surrogate endpoint as #aducanumab w/continued questions of its tie to patient harm & no AdComm beforehand: https://t.co/LQgpIgX6j4
— Reshma Ramachandran (@reshmagar) January 6, 2023
Another medication which was part of the accelerated approval pathway from the FDA, was Seagen Inc’s Tukysa (tucatinib) in combination with trastuzumab. Tukysa has already been prescribed to treat adults with a type of breast cancer called HER2 positive breast cancer, and it has now been approved for treating metastatic colorectal cancer. HCPs were very supportive overall for Tukysa entering a new therapy area. They celebrated it as another option for patients with colorectal cancer and were encouraging of the decision of the FDA.
Great day for GI and colorectal cancer!
FDA grants accelerated approval to tucatinib with trastuzumab for HER2+ colorectal cancer https://t.co/NPBWoGnIen#GI23 #colorectalcancer
— Suneel Kamath MD (@SKamath_MD) January 20, 2023
#Tucatinib just got #FDA approved for RAS WT HER-2 positive met #CRC #cancer. Another option for our patients!!!! Glad to have @VUMC_Cancer involved in this!!!👏🏻 #cancer #cancerresearch #ClinicalTrials @SeagenGlobal #mountaineer @KristenCiombor @VUMC_MD @VUMChealth @VUMCHemOnc
— Dr. Cathy Eng (@CathyEngMD) January 19, 2023
HCPs also shared their thoughts on NICE’s recommendation of Roche’s Polivy (polatuzumab vedotin) being used as part of a drug combination to treat adults with an aggressive form of blood cancer. The antibody drug conjugate was recommended to be used in combination with rituximab, cyclophosphamide, doxorubicin and prednisolone (R-CHP) for untreated diffuse large B-cell lymphoma (DLBCL). Haematologist and lymphoma specialist Graham Collins shared a thread offering his thoughts on the approval, speaking positively of the trial design but also noting the potentially high cost and limited evidence of PFS and OS benefit. Despite this he still declared that he would use the treatment as it offered the highest chance of cure even if there may be some negative consequences.
Pola-R-CHP has been approved by NICE for front line treatment of DLBCL in IPI 2-5. This means hospitals in England are legally obliged to offer it so this will become a new standard. More thoughts in thread. #lymsmhttps://t.co/aJLqntz4Jk pic.twitter.com/FEoQ8pWhyR
— Graham Collins (@graham74GC) January 25, 2023
Pola-R-CHP has been approved by NICE for front line treatment of DLBCL in IPI 2-5. This means hospitals in England are legally obliged to offer it so this will become a new standard. More thoughts in thread. #lymsmhttps://t.co/aJLqntz4Jk pic.twitter.com/FEoQ8pWhyR
— Graham Collins (@graham74GC) January 25, 2023
In late January, three more cancer treatments were approved by the FDA. Keytruda (Pembrolizumab) by Merck is an adjuvant drug targeting non-small cell lung cancer. HCPs were enthusiastic about the approval, citing it as ‘incredible news’.
Incredible news, that continues to benefit our patients with #lungcancer receiving life saving #Surgery@US_FDA approves pembrolizumab as adjuvant treatment for non-small cell lung #cancer https://t.co/0QbH7vCdt7 #LCSM #tssmn
— Dr. David Tom Cooke (@DavidCookeMD) January 27, 2023
Orserdu (Elacestrant) manufactured by Stemline Therapeutics, a subsidiary of Menarini Group also received FDA approval as a breast therapy treatment. HCPs celebrated the approval for broadening the range of medications on advanced or metastatic breast cancer and for coming earlier than expected.
We have another treatment option – and a couple of weeks earlier than expected!!!! #bcsm #MetastaticBreastCancer
FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer https://t.co/OUn6iGTa0b— Dr. Kelly Shanahan (@stage4kelly) January 28, 2023
Jaypirca (pirtobrutinib) by Eli Lilly was recently approved as part of the accelerated approval pathway for relapsed or refractory mantle cell lymphoma (MCL). HCPs were supportive of the important advancement for the treatment of relapsed/refractory mantle cell lymphoma quoting it as a ‘phenomenal drug’ and the approval as ‘big news for the MCL patients’.
Phenomenal drug. Happy to contribute patients into pirtobrutinib clinical trials @KantonsspitalLU @FDA grants accelerated approval to pirtobrutinib for relapsed or refractory mantle cell lymphoma https://t.co/5YHjzwE9Gz
— Thilo J. Zander (@ThiloZander) January 28, 2023
Big news for R/R #MCL pts
Great to see this approval. Here's hoping the EMA and MHRA will follow soon after.
Great to contribute to the BRUIN trial making this possible with @chanyooncheah @michaelwangmd
@niravshahmd + many others #MCL #lymsm #pirtobrutinib https://t.co/YYX6iu5oMq— Toby Eyre (@tobyeyre82) January 27, 2023
The three most shared links from HCPs discussing product launches in January were:
- An FDA press release on the accelerated approval of Leqembi (lecanemab-irmb) for alzheimers disease
- An FDA press release on the approval of pembrolizumab as adjuvant treatment for non-small cell lung cancer
- A Statnews article about the accelerated approval of Leqembi (lecanemab-irmb) for alzheimers disease
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 January and 31 January 2023 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 January and 31 January 2023, there were 2,916 HCP mentions of new pharmaceutical product launches and drug approvals from 1,954 unique HCP authors from around the world.
Click here to view the latest product tracker
 By Paul Cranston
By Paul Cranston 
