05.01.2024 | Tracker
Product Launch Tracker: Hundreds of eHCPs are enthusiastic about the first Sickle Cell Disease gene therapies.
Every month, CREATION.co’s tracking updates bring you the latest insights from the online conversation of healthcare professionals (eHCPs) across the globe discussing product launches. Discover which new drug approvals eHCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout December 2023 CREATION.co tracked the global conversations of 1,854 eHCPs who posted 2,732 English-language X (formerly Twitter) posts about the launches and approvals of new products.
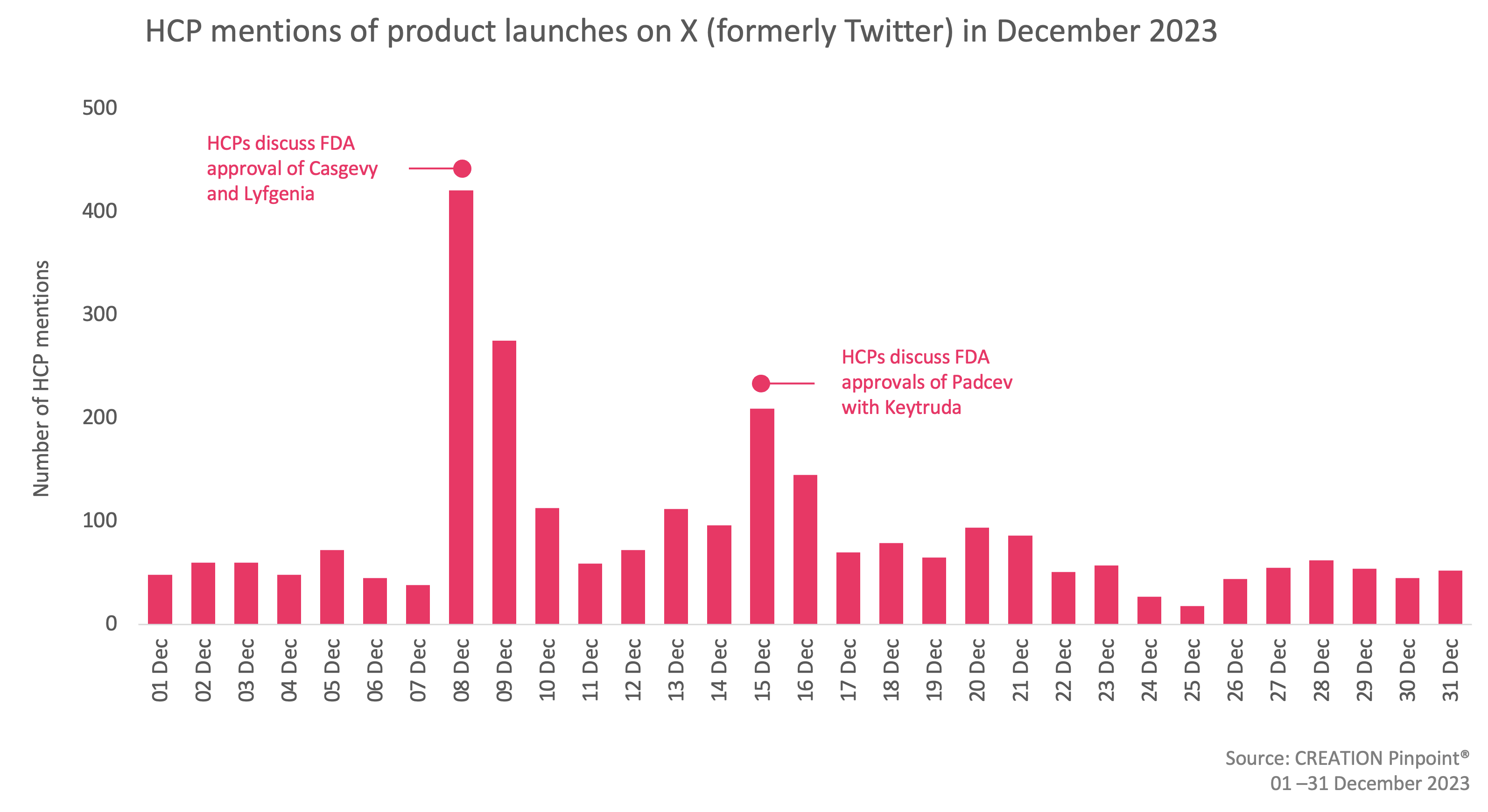
In December, 221 more eHCPs discussed treatment approvals compared to November and there were 16% more eHCP mentions of new pharmaceutical product launches and drug approvals.
The approvals which have received the most traction occurred on 8 December, when the FDA approved two gene therapies for the treatment of sickle cell disease. Two milestone treatments, Casgevy and Lyfgenia, represent the first cell-based gene therapies for the treatment of sickle cell disease (SCD). Vertex and CRISPR Therapeutics’ Casgevy is the first FDA-approved therapy utilising CRISPR/Cas9, a type of genome editing technology and Bluebird Bio’s Lyfgenia is a cell-based gene therapy. eHCPs were thrilled about the approvals, calling them “historic medical milestones” and expressed the belief that “a new era of CRISPR-based medicines” is about to begin, bringing great medical potential.
eHCPs showed an equal level of excitement about the news for both treatments, highlighting the fact that Casgevy is using the CRISPR technology and saying that “medicine will never be the same again”.
BIG NEWS!!! This new treatment will save the lives of so many patients with SS, which disproportionately affects individuals of African ancestry ⚕️| In historic decision, FDA approves a CRISPR-based medicine for treatment of sickle cell disease https://t.co/48JF5q1jjb
— Jared Boyce (@Jared_Boyce) December 8, 2023
First #genetherapies approved for #SickleCellDisease!! These are transformative therapies that will help so many people!!
These inspire hope for people living with #SCD. This is not the end-it’s the beginning!!!https://t.co/ZseZkLrQBV— Julie Kanter (@jkw4444) December 8, 2023
On 15 December, the FDA approved Astellas’ Padcev in combination with Merck’s Keytruda, for patients with locally advanced or metastatic urothelial cancer. eHCPs applauded the approval as it will transform the lives of the patients by posting that 15 December was a big day for bladder cancer patients and characterising the approval as a breakthrough cancer treatment, representing “unprecedented times” and they congratulated the research team for their effort and scientific contribution.
Great news for our #bladdercancer patients!
On December 15, 2023, the Food and Drug Administration (FDA) approved enfortumab vedotin-ejfv (Padcev, Astellas Pharma) in combination with pembrolizumab (Keytruda, Merck) for patients with locally advanced or metastatic urothelial… pic.twitter.com/e3jFHFqMUa
— Ashish M. Kamat, MD, MBBS (@UroDocAsh) December 15, 2023
The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) ≥10: a decision centered on empowering patients and their physicians – Annals of Oncology @Annals_Oncology https://t.co/qxalZ9vsSq
— Vivek Subbiah, MD (@VivekSubbiah) December 7, 2023
The three most shared stories from eHCPs discussing product launches in December were:
- An FDA press release on the approval of Casgevy and Lyfgenia, the first cell-based gene therapies for the treatment of sickle cell disease.
- A STAT news article on the approval of Casgevy, the first CRISPR-based medicine for treatment of sickle cell disease.
- An FDA press release on the approval of Padcev with Keytruda for locally advanced or metastatic urothelial cancer.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language X conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 December and 31 December 2023 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their X conversations.
Between 1 December and 31 December 2023, there were 2,732 eHCP mentions of new pharmaceutical product launches and drug approvals from 1,854 unique eHCP authors from around the world.
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou