Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
Throughout June 2023 we tracked the global conversations of 1,949 eHCPs who posted 2,817 English-language Twitter posts about the launches and approvals of new products.
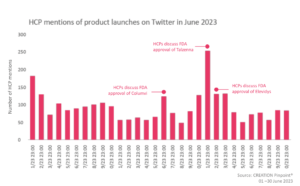
In June, several product approvals were discussed online by HCPs. They offered their thoughts on the first ever gene therapy treatment for DMD, as well as sharing their enthusiasm about the approval of Talzenna, a new therapy shaping the future of prostate cancer.
During June, the FDA approved Elevidys by Sarepta Therapeutics, the first gene therapy for the treatment of paediatric patients four to five years of age with Duchenne muscular dystrophy (DMD). eHCPs were on the fence regarding this accelerated approval as they discussed some limitations in the trial’s results, and several clinical professionals did not agree with the decision. However, a number of eHCPs were enthusiastic about the approval calling it a “groundbreaking treatment”.
FDA grants accelerated approval to first gene therapy for Duchenne muscular dystrophy, despite internal objections – @Nature #drugdiscovery #DMD #Duchenne #neuroscience https://t.co/74FEMq9xnM
— Virginie Buggia-Prevot (@vbprevot) June 23, 2023
Hope for kids suffering from Duchenne Muscular Dystrophy. A new drug is being approved by the U.S. FDA. Hopefully, it will alleviate the pains of kids suffering from this incapacitating disorder 🙏💙 https://t.co/tDpLqSIory
— Vera K. Ghali, MD (@veraghali) June 23, 2023
On 20 June, the FDA announced the approval of Pfizer’s Talzenna (talazoparib), an oral poly ADP-ribose polymerase (PARP) inhibitor, in combination with Xtandi (enzalutamide), for the treatment of adult patients with homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC). eHCPs were very pleased to see this treatment approved, as they commented on the remarkable results of the clinical trial and labelled it as a significant leap forward in prostate cancer treatment.
Hear ye hear ye! 🔥🔥
FDA Approves Talazoparib for Metastatic Prostate Cancer together with Enzalutamide for patients with homologous repair defects https://t.co/yLzQ22gwbK— Razelle Kurzrock, MD (@Dr_R_Kurzrock) June 22, 2023
⭐️Great news for patients with prostate cancer! The US FDA has approved Talazoparib+ Enzalutamide for HRR+ mCRPC! Congratulations to @neerajaiims et al for spearheading this trial & bringing a new treatment option to the clinic. #prostatecancer
Knew this baby would graduate right… https://t.co/AKxYdFvYGQ pic.twitter.com/UKyiIYqli5— Vivek Subbiah, MD (@VivekSubbiah) June 21, 2023
On 15 June, the FDA has granted accelerated approval to Genentech’s Columvi (glofitamab-gxbm) as a therapy for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) or large B-cell lymphoma arising from follicular lymphoma. eHCPs called the day a historical one for DLBCL and were rather excited about the treatment. They quoted “great news for patients” and hoped to be approved in other countries soon.
Great news! #17ICML
Glofitamab approved for R/R DLBCL after ≥2 lines of therapy
Congrats @mike_dickinson1 for his leadership of many of the clinical trials needed to get to this accelerated approval
And kudos to @genentech for a fixed duration regimenhttps://t.co/wI6YYQMjxY
— Eddie Cliff (@Eddie_Cliff) June 16, 2023
Good news for DLBCL patients. https://t.co/VFWxR4byXT
— Daniel Molin (@Dr_Daniel_Molin) June 16, 2023
On 20 June, the FDA approved the first anti-inflammatory drug for reducing cardiovascular events among adults who have established atherosclerotic cardiovascular disease (ASCVD) under the name Lodoco (colchicine). AGEPHA Pharma’s drug reduces the risk of cardiac event risk by an additional 31%. There was a small number of eHCPs who were concerned about the possibility of a price increase for generic colchicine. However, overall, eHCPs were quick to recommend the new treatment online, as an effective option, calling the approval “a new era” in residual inflammatory ASCVD risk.
Breaking news: first anti-inflammatory therapy with colchicine is approved by FDA to reduce CV risk!! The dawn of a new era in CV medicine! https://t.co/sqEFgArA6h
— Konstantinos Stellos, MD (@K_Stellos) June 20, 2023
FDA has approved colchicine for CVD prevention. Lodoco dose is 0.5mg. Pricing likely to be high for new approval for this formulation. For gout patients with CVD/risk, this may change recommendations re: duration of colchicine “prophylaxis”. @G_CANgout https://t.co/TGjkU4ungz
— Tuhina Neogi, MD, PhD (@Tuhina_Neogi) June 23, 2023
The three most shared links from HCPs discussing product launches in June were:
- A TCTMD news article on FDA approval of Lodoco (colchicine) for decreasing cardiovascular events.
- An OncLive news article on FDA approval of Columvi (Glofitamab-gxbm) for Relapsed/Refractory DLBCL.
- A Pfizer article on the FDA press release on the accelerated approval to Talzenna for metastatic castration-resistant prostate cancer (mCRPC).
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 June and 30 June 2023 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their Twitter conversations.
- Between 1 June and 30 June 2023, there were 2,817 eHCP mentions of new pharmaceutical product launches and drug approvals from 1,949 unique eHCP authors from around the world.
Click here to read the latest Product launch tracker
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou 


