01.09.2023 | Tracker
Product Launch Tracker: eHCPs thrilled about approval of first oral treatment for postpartum depression
Every month, CREATION.co’s tracking updates bring you the latest insights from the conversation of healthcare professionals (HCPs) across the globe discussing product launches. Discover which new drug approvals HCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
Throughout August 2023 we tracked the global conversations of 1,889 eHCPs who posted 2,896 English-language X (formerly Twitter) posts about the launches and approvals of new products.
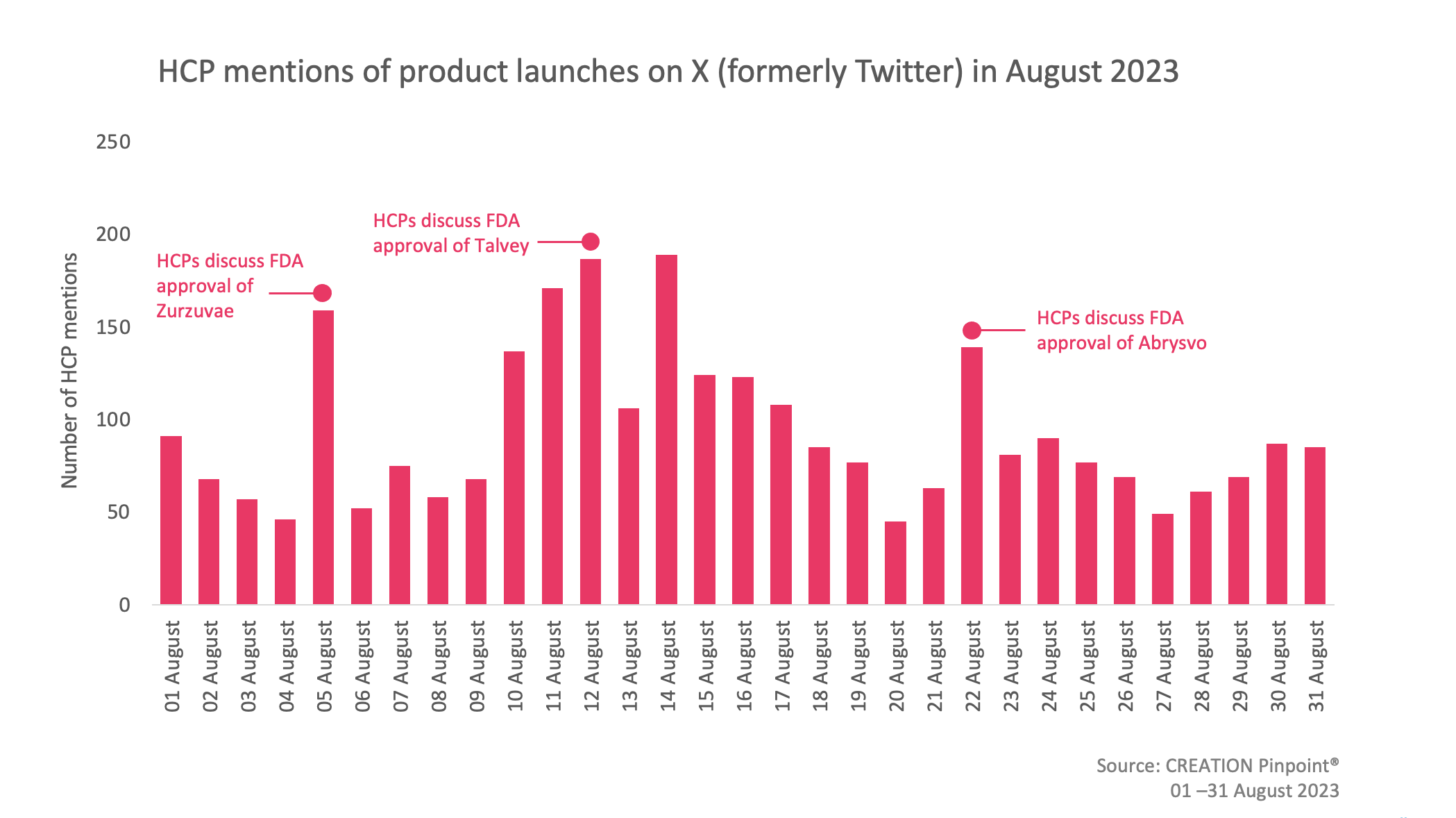
In August, slightly more eHCPs discussed treatment approvals compared to the previous month. eHCPs offered their thoughts on the approval of Abrysvo, an RSV vaccine which is administered to pregnant women to protect infants from birth, as well as Zurzuvae, a postpartum depression treatment.
On 04 August, the FDA granted full approval to Zurzuvae (zuranolone), the first oral treatment for postpartum depression by Sage Therapeutics. Up until now, the only way to receive treatment for postpartum depression was via an IV injection administered in healthcare facilities. eHCPs were delighted with the approval, with a few mentioning the impressive and speedy results of the novel treatment. eHCPs referred to it as a “potential milestone” and were excited for the expansion of their options.
For the First Time, There’s a Pill for Postpartum Depression:
Because the pill works faster than other antidepressants and is taken for only two weeks, it may encourage more treatment of the debilitating condition.https://t.co/ojIMi3uNtE— Robyn Brickel (@RobynBrickel) August 5, 2023
This is huge news 🙌🏽
FDA approves first postpartum depression drug, Zurzuvae – The Washington Post https://t.co/AURe4QUEaW
— Sulman Aziz Mirza, MD? | TheKicksShrink (@sulmoney) August 5, 2023
On 10 August, the FDA announced the approval of Talvey (talquetamab) by Janssen, a first in class specific therapy for the treatment of patients with heavily pretreated multiple myeloma. eHCPs shared the news mentioning that they are excited for having another safe and effective treatment available. Certain eHCPs were particularly excited about the approval describing the treatment as “great for patients”.
1/ 5 years ago: "GPRC5D is an orphan receptor with no known ligand."
Today: "Time to use our FDA-approved ligand to kick some myeloma butt!"
Wonderful to see talquetamab now approved in R/R #MMsm. Esp important after BCMA failure. Congrats @JanssenGlobal and MonumenTAL teams! https://t.co/UKCyfYy5ZF
— Rahul Banerjee, MD, FACP (@RahulBanerjeeMD) August 10, 2023
The FDA approval of talquetamab is great news- having a commercially available new class of drugs for refractory disease is a win.
The taste changes (weight loss that puts ozempic to shame), and skin toxicity may limit uptake/enthusiasm in earlier lines. Time will tell#mmsm
— Manni Mohyuddin (@ManniMD1) August 10, 2023
On 21 August, the FDA granted approval to Abrysvo, a single dose vaccine for the prevention of Respiratory Syncytial Virus (RSV). It is the first vaccine approved for use in pregnant individuals to prevent lower respiratory tract disease (LRTD) and severe LRTD caused RSV in infants from birth through 6 months of age. eHCPs shared the approval with their peers commenting on the fact that it protects infants from birth. A number of eHCPs were thrilled about the approval calling it ‘great news’ for preventing infants suffering.
🔥US FDA approved Abrysvo (Respiratory Syncytial Virus Vaccine),the first vaccine approved for use in pregnant individuals to prevent lower respiratory tract disease & severe LRTD caused by RSV in infants from birth through 6 months of age🔥https://t.co/9Lkhfgmbfx
— Antibiotic Steward Bassam Ghanem 🅱️C🆔🅿️🌟 (@ABsteward) August 21, 2023
This is great news! #RSV is a serious cause of respiratory issues in young infants. Vaccinating pregnant mothers prior to birth will give babies #RSV antibodies and passive immunity for up to 6 months! #PedsICU #Neotwitter #Tweetiatrician #VaccinesWork https://t.co/qVEofe4ka9
— John Daniel, MD, MS (@JohnDanielMD) August 22, 2023
The three most shared links from eHCPs discussing product launches in August were:
- A PR Newswire news article on FDA approval of Talvey, a first-in-class treatment for multiple myeloma.
- A BMJ article on how brexpiprazole, an antipsychotic FDA approved treatment failed to show clinical benefits and increased mortality.
- An OncoAlert newsletter which captured the EMA approval of Keytruda in Gastric Cancer during July.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal or review all previous editions of the Product Launch Tracker.
Methodology
- Using CREATION Pinpoint® the English-language X conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 August and 31 August 2023 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their X conversations.
- Between 1 August and 31 August 2023, there were 2,896 eHCP mentions of new pharmaceutical product launches and drug approvals from 1,889 unique eHCP authors from around the world.
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou 

