01.02.2024 | Tracker
Product Launch Tracker: eHCPs celebrate approval in cervical cancer
Every month, CREATION.co’s tracking updates bring you the latest insights from the online conversation of healthcare professionals (eHCPs) across the globe discussing product launches. Discover which new drug approvals eHCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout January 2024 CREATION.co tracked the global conversations of 1,255 eHCPs who posted 1,745 English-language X (formerly Twitter) posts about the launches and approvals of new products.
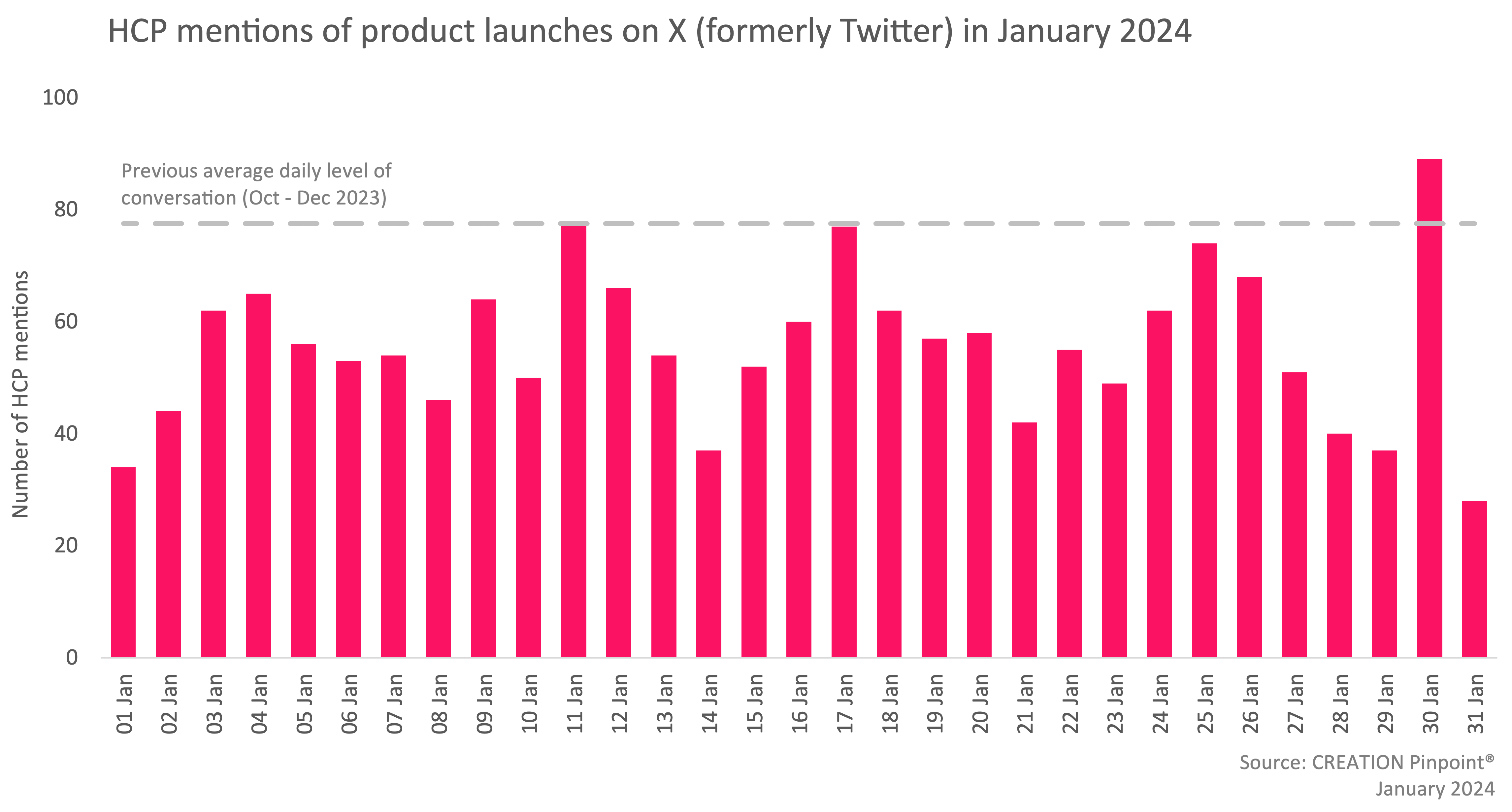
In January, 573 fewer eHCPs discussed treatment approvals compared to December and there were 35% fewer mentions of new pharmaceutical product launches and drug approvals. However, two stories received eHCPs’ attention.
On 12 January, the FDA approved Merck’s Keutruda (pembrolizumab) in combination with chemoradiotherapy, for patients with late-stage cervical cancer. eHCPs expressed optimism regarding the approval, citing the significance of the 18-year gap since the last approval for the combination of pembrolizumab and chemotherapy as a treatment option. A small number of eHCPs characterised the results of this trial assessing this treatment as practice-changing and establishing a new standard of care in cervical cancer treatment.
~40% of pts with LA #CervicalCancer will relapse after cCRT. The practice-changing results of KN-A18 led to the recent FDA approval of Pembrolizumab + cCRT, which now represents the new SoC after 2 decades! #Prevention, though, remains the best treatment! @OncoAlert pic.twitter.com/iPeIVWT6QX
— Oraianthi Fiste (@OraianthiF) January 14, 2024
We must educate and rethink, 18 yrs since last FDA approval of a drug in combo w/ RT. Pembro+CRT in Cervical CA 👍! Today at @theNCI Dr. Kiran from @JNJNews spent several slides talking about our own @NiuSanford recent @JCO_ASCO paper re disparities in RT trial expectations. pic.twitter.com/h5v6IMnvUM
— Todd Aguilera MD PhD (@aguilera_md) January 17, 2024
On 17 January, the FDA approved the first generic of Dificid (fidaxomicin), an antibiotic used to treat an infection called Clostridioides difficile – associated diarrhoea. eHCPs responded with great enthusiasm upon receiving the news of the treatment approval. Some saw the approval as the start of an important year in infectious diseases. Their conviction derived from the perceived simplification of medication access following this FDA approval.
I can say now that the year 2024 is going to be 🔥 in infectious diseases
ACCESS RCT
SIMPLIFY RCT
And now
SABATO RCT
And US FDA approves Fidaxomicin first generic
What's coming NeXt?#IDXposts https://t.co/4cIYbPDkfZ— Antibiotic Steward Bassam Ghanem 🅱️C🆔🅿️🌟 (@ABsteward) January 18, 2024
Great news!!
🔥 US FDA approves the first generic of Dificid (fidaxomicin)🔥#idxposts https://t.co/LBtCuAvdqj— Antibiotic Steward Bassam Ghanem 🅱️C🆔🅿️🌟 (@ABsteward) January 17, 2024
The two most shared stories from eHCPs discussing product launches in January were:
- An FDA press release on the approval of pembrolizumab with chemoradiotherapy for Stage III-IVA cervical cancer.
- An FDA data access release on the approval of the first generic of Deficid for the treatment of Clostridioides difficile – associated diarrhoea.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language X conversations of eHCPs globally discussing new pharmaceutical product launches and drug approvals between 1 January and 31 January 2024 were analysed in order to discover which new product launches eHCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by eHCPs in their X conversations.
- Between 1 January and 31 January 2024, there were 2,732 eHCP mentions of new pharmaceutical product launches and drug approvals from 1,854 unique eHCP authors from around the world.
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou